In this post, I link to and excerpt from 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association [PubMed Citation] [Full-Text HTML] [Full-Text PDF]. Stroke. 2021 Jul;52(7):e364-e467.
All that follows is from the above resource.
- TOP 10 Take-Home Messages for the Secondary Stroke Prevention Guideline
- Preamble
- 1. INTRODUCTION
- 2. General Concepts
- 3. Diagnostic Evaluation for Secondary Stroke Prevention
- 4. Vascular Risk Factor Management
- 4.3. Treatment of Hyperlipidemia for Secondary Prevention of Stroke
- 5. Management by Etiology
- 6. Systems of Care for Secondary Prevention
- AHA Stroke Council Scientific Statement Oversight Committee
- Disclosures
TOP 10 Take-Home Messages for the Secondary Stroke Prevention Guideline
Specific recommendations for prevention strategies often depend on the ischemic stroke/transient ischemic attack subtype. Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke etiology (when possible), and to identify targets for treatment in order to reduce the risk of recurrent ischemic stroke. Recommendations are now grouped by etiologic subtype.
Management of vascular risk factors remains extremely important in secondary stroke prevention, including (but not limited to) diabetes, smoking cessation, lipids, and especially hypertension. Intensive medical management, often performed by multidisciplinary teams, is usually best, with goals of therapy tailored to the individual patient.
Lifestyle factors, including healthy diet and physical activity, are important for preventing a second stroke. Low-salt and Mediterranean diets are recommended for stroke risk reduction. Patients with stroke are especially at risk for sedentary and prolonged sitting behaviors, and they should be encouraged to perform physical activity in a supervised and safe manner.
Changing patient behaviors such as diet, exercise, and medication compliance requires more than just simple advice or a brochure from their physician. Programs that use theoretical models of behavior change, proven techniques, and multidisciplinary support are needed.
Antithrombotic therapy, including antiplatelet or anticoagulant agents, is recommended for nearly all patients without contraindications. With very few exceptions, the combination of antiplatelets and anticoagulation is typically not indicated for secondary stroke prevention. Dual antiplatelet therapy is not recommended long term, and short term, dual antiplatelet therapy is recommended only in very specific patients, including those with early arriving minor stroke and high-risk transient ischemic attack or severe symptomatic intracranial stenosis.
Atrial fibrillation remains a common and high-risk condition for second ischemic stroke. Anticoagulation is usually recommended if the patient has no contraindications. Heart rhythm monitoring for occult atrial fibrillation is usually recommended if no other cause of stroke is discovered.
Extracranial carotid artery disease is an important and treatable cause of stroke. Patients with severe stenosis ipsilateral to a nondisabling stroke or transient ischemic attack who are candidates for intervention should have the stenosis fixed, likely relatively early after their ischemic stroke. The choice between carotid endarterectomy and carotid artery stenting should be driven by specific patient comorbidities and features of their vascular anatomy.
Patients with severe intracranial stenosis in the vascular territory of ischemic stroke or transient ischemic attack should not receive angioplasty and stenting as a first-line therapy for preventing recurrence. Aggressive medical management of risk factors and short-term dual antiplatelet therapy are preferred.
There have been several studies evaluating secondary stroke prevention of patent foramen ovale closure since the previous guideline in 2014. It is now considered reasonable to percutaneously close patent foramen ovale in patients who meet each of the following criteria: age 18–60 years, nonlacunar stroke, no other identified cause, and high risk patent foramen ovale features.
Patients with embolic stroke of uncertain source should not be treated empirically with anticoagulants or ticagrelor because it was found to be of no benefit
Each year, ≈795 000 individuals in the United States experience a stroke, of which 87% (690 000) are ischemic and 185 000 are recurrent.1 Approximately 240 000 individuals experience a transient ischemic attack (TIA) each year.2 The risk of recurrent stroke or TIA is high but can be mitigated with appropriate secondary stroke prevention. In fact, cohort studies have shown a reduction in recurrent stroke and TIA rates in recent years as secondary stroke prevention strategies have improved.3,4 A meta-analysis of randomized controlled trials (RCTs) of secondary stroke prevention therapies published from 1960 to 2009 showed a reduction in annual stroke recurrence from 8.7% in the 1960s to 5.0% in the 2000s, with the reduction driven largely by improved blood pressure (BP) control and use of antiplatelet therapy.5 The changes may have been influenced by changes in diagnostic criteria and differing sensitivities of diagnostic tests over the years.
The overwhelming majority of strokes can be prevented through BP control, a healthy diet, regular physical activity, and smoking cessation. In fact, 5 factors—BP, diet, physical inactivity, smoking, and abdominal obesity—accounted for 82% and 90% of the population-attributable risk (PAR) for ischemic and hemorrhagic stroke in the INTERSTROKE study (Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries).5a Similarly, the Global Burden of Disease Study showed that 90.5% (95% uncertainty interval, 88.5–92.2) of the global burden of stroke was attributable to modifiable risk factors.6 A modeling study showed that targeting multiple risk factors has additive benefits for secondary prevention; specifically, aspirin, statin, and antihypertensive medications, combined with diet modification and exercise, can result in an 80% cumulative risk reduction in recurrent vascular events.7 Although the benefits of a healthy lifestyle and vascular risk factor control are well documented,8,9 risk factors remain poorly controlled among stroke survivors.10–14
1.4. Scope of the Guideline
The aim of the present guideline is to provide clinicians with evidence-based recommendations for the prevention of future stroke among survivors of ischemic stroke or TIA. It should be noted that this guideline does not cover the following topics, which have been addressed elsewhere:
Acute management decisions (covered in the “2019 Update to the 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke”16),
Intracerebral hemorrhage (ICH; covered in the “Guidelines for the Management of Spontaneous Intracerebral Hemorrhage”17),
Primary prevention (covered in the “Guidelines for the Primary Prevention of Stroke”18 and “2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease”19),
Special considerations for stroke prevention in women (covered in the “Guidelines for the Prevention of Stroke in Women”20), and
Cerebral venous sinus thrombosis (covered in “Diagnosis and Management of Cerebral Venous Thrombosis”22).
In general, with very few exceptions, the literature supports the concept that patients with TIA and those with ischemic stroke should be treated the same in terms of secondary prevention.
This guideline is divided into 4 sections:
Diagnostic Evaluation for Secondary Stroke Prevention
Vascular Risk Factor Management
Management by Etiology
Systems of Care for Secondary Ischemic Stroke Prevention.
2. General Concepts
2.1. Definitions
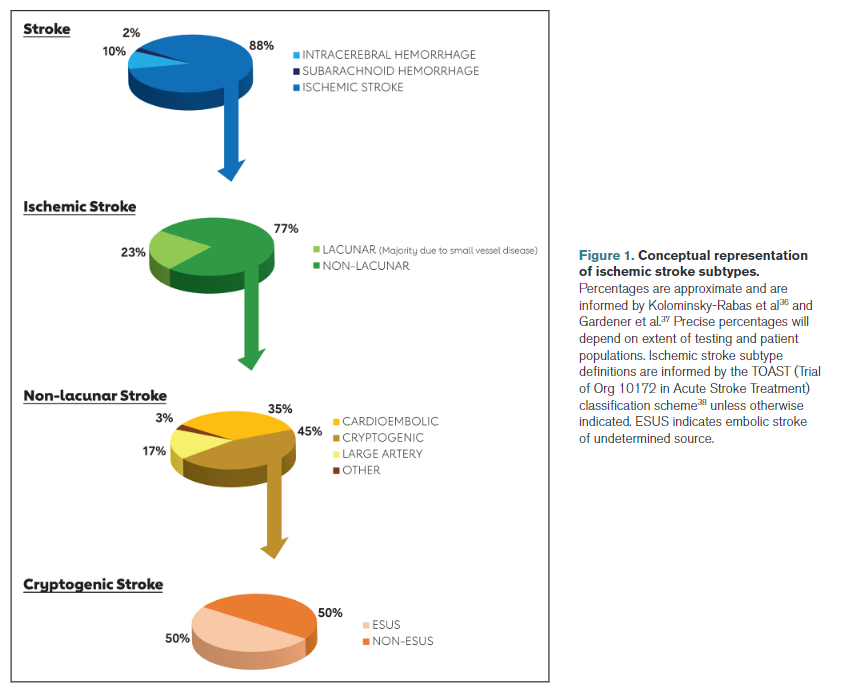
Lacunar stroke: Lacunar syndrome, with normal computed tomography (CT)/magnetic resonance imaging (MRI) or subcortical stroke measuring <1.5 cm in diameter on CT or MRI. Most, although not all, of lacunar strokes are due to small vessel disease.
Stroke attributable to small vessel disease: Subcortical stroke measuring <1.5 cm in diameter on CT or MRI without evidence of a concomitant cortical infarct.
Cardioembolic stroke: Stroke attributable to arterial occlusion from an embolus that presumably arose in the heart. Clinical and brain imaging findings are similar to those described in large artery atherosclerosis. Evidence of a previous TIA or stroke in >1 vascular territory supports a clinical diagnosis of cardioembolic stroke.
Cryptogenic stroke: An imaging-confirmed stroke with unknown source despite thorough diagnostic assessment (including, at a minimum, arterial imaging, echocardiography, extended rhythm monitoring, and key laboratory studies such as a lipid profile and hemoglobin A1c [HbA1c]).
Stroke caused by large artery atherosclerosis: Ischemic stroke in the vascular distribution of a major intracranial or extracranial artery with >50% stenosis or occlusion on vascular imaging. Clinical findings include those of cerebral cortical involvement or brainstem or cerebellar dysfunction. Cortical and cerebellar lesions and brainstem or subcortical lesions >1.5 cm are considered potentially caused by large artery atherosclerosis. Diagnostic studies should exclude potential sources of cardioembolic embolism.
ESUS: A stroke that appears nonlacunar on neuroimaging without an obvious source after a minimum standard evaluation (including arterial imaging, echocardiography, extended rhythm monitoring, and key laboratory studies such as a lipid profile and HbA1c) to rule out known stroke etiologies such as cardioembolic sources and atherosclerosis proximal to the stroke.39
A diagnosis of ESUS implies that the stroke is embolic in origin, given the nonlacunar location; however, the source of the embolus is unknown, despite a minimal standard evaluation. Although cryptogenic stroke similarly implies that the cause of the origin is unknown, the stroke is not necessarily embolic. Individuals with ESUS have cryptogenic stroke, but the converse is not always the case.
2.3. Contraindications
Treatment should always be tailored to patients’ individual situations. Therefore, as a rule, we did not include the statement “unless contraindicated” in the recommendations. It is implicit that if a recommendation is contraindicated in a patient’s circumstance, it should not be implemented.
2.5. Antithrombotic Dosing
Unless stated otherwise in the recommendations herein, the international normalized ratio (INR) goal for warfarin is 2.0 to 3.0 and the dose of aspirin is 81 to 325 mg.
2.6. Application Across Populations
Unless otherwise indicated, the recommendations in this guideline apply across race/ethnicity, sex, and age groups. Special considerations to address health equity are delineated in section 6.3, Health Equity.
3. Diagnostic Evaluation for Secondary Stroke Prevention
Synopsis
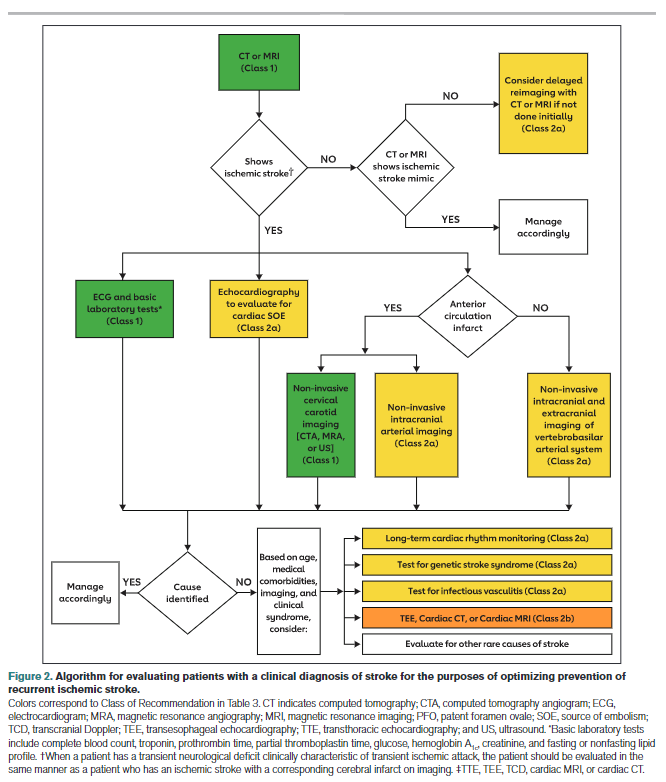
Patients presenting with signs and symptoms of acute stroke will undergo an evaluation tailored to ensure that, when appropriate, they receive reperfusion therapy (Figure 2). Imaging recommendations based on acute treatment considerations overlap with, but are not identical to, imaging recommendations based on secondary stroke prevention considerations. Recommendations presented in this guideline focus
on evaluations done for the purposes of confirming the diagnosis of stroke and characterizing its pathomechanism by identifying potential sources of cardioembolism, thromboembolism from large artery atherosclerosis, dissection, or other disease processes such as hypercoagulability. Confirmation of stroke diagnosis may require follow-up head imaging because of poor sensitivity of noncontrast CT for small or hyperacute infarcts. Some conditions associated with stroke and with specific therapies are common (eg, AF), whereas others are relatively rare (eg, endocarditis). The variable yield of testing means that treating physicians need to exercise judgment on the likelihood that a test will alter management in a given clinical situation.
Recommendation-Specific Supportive Text
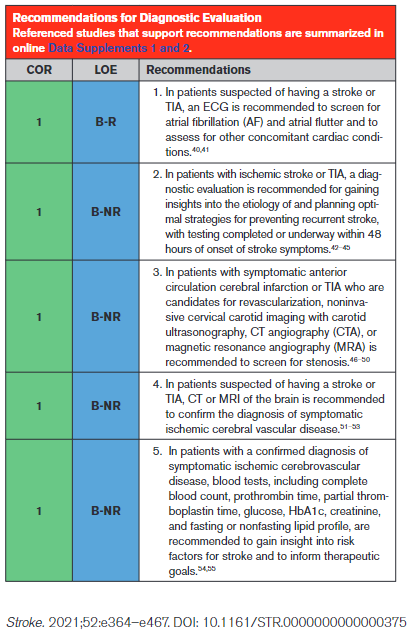
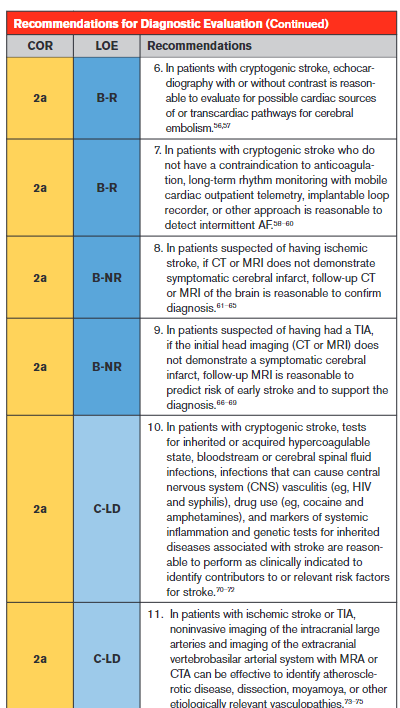
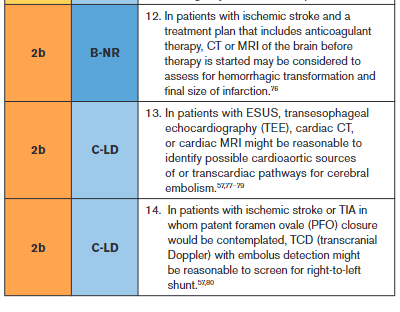
An ECG is a simple, noninvasive means of diagnosing AF in patients with acute stroke. A meta-analysis through 2014 found that the proportion of patients diagnosed with poststroke AF in the emergency department by electrocardiography was 7.7% (95% CI, 5.0–10.8).40 An ECG can also detect pertinent comorbidities that may have therapeutic implications. About 3% of patients presenting with acute stroke also have acute myocardial infarction (MI).41
Effective secondary prevention requires timely evaluation of stroke mechanism, with the intent of identifying modifiable risk factors. The risk of recurrent stroke in the short term and long term varies by stroke mechanism.42–45 The risk of stroke within 90 days after a first stroke is ≈5%, but the risk can vary greatly from >10% to <1%, depending in part on mechanism.42 Symptomatic carotid stenosis and AF are important to diagnose in a timely fashion to allow implementation of specific treatments with proven efficacy.
Because patients with symptomatic high-grade cervical carotid stenosis are candidates for revascularization, it is appropriate to screen for stenosis in any patient who may have such stenosis. Initial testing for carotid stenosis should be done with a noninvasive test such as CTA, MRA, or ultrasonography rather than digital subtraction angiography, with case series finding a risk of stroke ranging from 0.3% to 3.0%.81–83 Experienced stroke centers typically have a risk of stroke attributable to digital subtraction angiography of <0.5%. For patients at high risk of carotid artery stenosis who can undergo surgery without delay, immediate CTA is the most cost-effective strategy.84 Using consensus interpretation criteria, carotid ultrasonography has a sensitivity of 38.8%, specificity of 91.6%, and accuracy of 87.1% for ≥70% stenosis.47 With the use of a 70% cutoff value for carotid stenosis, CTA and digital subtraction angiography were in agreement in 78 of 81 vessels (95% CI, 90–99) in a series of patients with stroke or TIA.48 Compared with digital subtraction angiography, a meta-analysis of studies performed in 2008 found the overall sensitivity of time-of-flight MRA for the detection of 70% to 99% internal carotid artery (ICA) stenoses to be 91.2% with a specificity of 88.3%, whereas the sensitivity of contrast-enhanced MRA was 94.6% with a specificity of 91.9%.49
An accurate diagnosis of ischemic stroke or TIA is essential for justifying and optimizing stroke prevention. Many patients will have had brain imaging in the acute setting to exclude stroke mimics and to include stroke “chameleons” (stroke initially thought to be an alternative diagnosis). About 15% to 25% of patients thought to have stroke on clinical grounds will be given an alternative diagnosis with the help of brain imaging.51 About 13% of patients with stroke or TIA thought to have a nonstroke diagnosis for their neurological symptoms will be given the diagnosis of stroke with the help of brain imaging.52,53 A prospective, multicenter multinational study showed that, in patients with recent minor focal nonmotor, nonspeech neurological deficits, diffusion-weighted MRI detected acute infarction in 13.5% and that this finding had prognostic relevance because detection of infarction was associated with a >6-fold increase in the risk of recurrent stroke at 1 year.85
As reported in this guideline, control of hypertension (Section 4.2), blood glucose (Section 4.4), and lipids (Section 4.3) have been proven effective for reducing the risk of ischemic stroke; thus, assessment of whether the patient is at therapeutic goal for these metabolic parameters helps to optimize therapy. Fasting is not routinely required for lipid testing because the lipid profile components under fasting and nonfasting conditions differ in nonclinically significant degrees (the exception being patients with nonfasting triglycerides of >440 mg/dL, who should have fasting levels drawn).86 HbA1c determination can detect new cases of type 2 diabetes (T2D) in ≈11.5% of patients presenting with acute ischemic stroke and prediabetes in 36.2%.54 Abnormal blood testing can help to stratify risk so that physicians can concentrate efforts of prevention on those at highest risk. In patients with lacunar infarction, chronic kidney disease is associated with a 50% increase in risk of recurrent stroke.55 Testing prothrombin time and activated partial thromboplastin time screens for diverse clotting and bleeding disorders that are relevant to active management of patients with acute stroke. An isolated prolonged activated partial thromboplastin time can be seen with heparin use, lupus anticoagulant, or clotting factor deficiencies.87 All of these states or exposures are relevant to long-term management of patients with stroke. Liver failure, malnutrition, malabsorption, myeloproliferative diseases, and disseminated intravascular coagulation can cause acquired factor deficiencies and would have relevance in managing patients with stroke.88