The Web Tables below are excerpts from Resource 2 below, 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure – Web Addenda [Full Text PDF -you may need t register for a free ESC account to access this resource]. Other text and tables are from Resource 1 below:
7.2 Treatments recommended in all symptomatic patients with heart failure with reduced ejection fraction
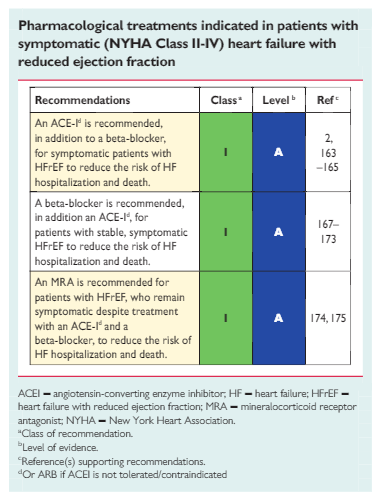
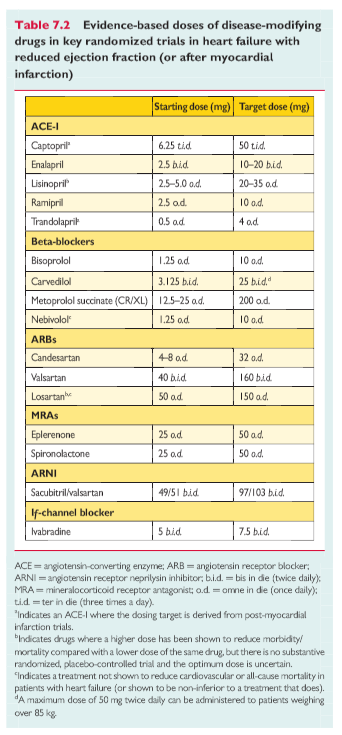
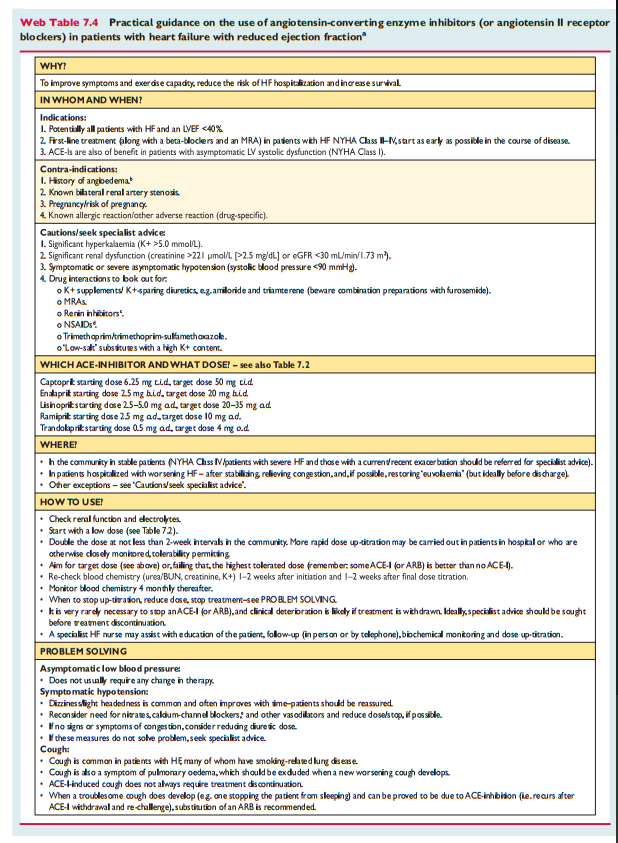
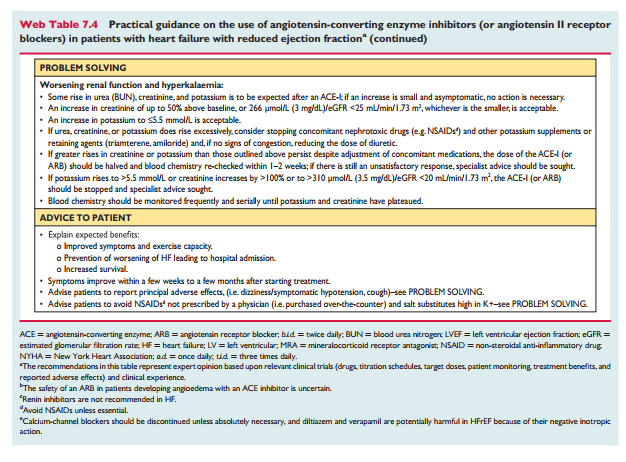
7.2.1 Angiotensin-converting enzyme inhibitorsACEIs have been shown to reduce mortality and morbidity in pa- tients with HFrEF 2,5,163 – 165 and are recommended unless contrain-dicated or not tolerated in all symptomatic patients. ACEIs should be up-titrated to the maximum tolerated dose in order to achieve adequate inhibition of the renin angiotensin –aldosterone system (RAAS). There is evidence that in clinical practice the majority of patients receive suboptimal doses of ACEI. 166 ACEIs are also recommended in patients with asymptomatic LV systolic dysfunction to reduce the risk of HF development, HF hospitalization and death (see Section 6).Practical guidance on how to use ACE inhibitors is given in Web Table 7.4
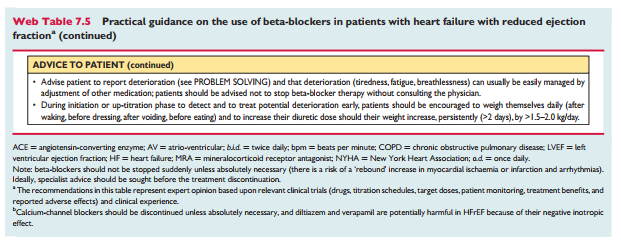
7.2.2 Beta-blockersBeta-blockers reduce mortality and morbidity in symptomatic patients with HFrEF, despite treatment with an ACEI and, in most cases, a diuretic, 167,168,170,172,173 but have not been tested in congested or decompensated patients. There is consensus that beta-blockers and ACEIs are complementary, and can bestarted together as soon as the diagnosis of HFrEF is made. There is no evidence favouring the initiation of treatmentwith a beta-blocker before an ACEI has been started. 176 Beta blockers should be initiated in clinically stable patients at a low dose and gradually up-titrated to the maximum tolerated dose. In patients admitted due to acute HF (AHF) beta-blockers should be cautiously initiated in hospital, once the patient is stabilized.An individual patient data meta-analysis of all the major beta-blocker trials in HFrEF has shown no benefit on hospital admis-sions and mortality in the subgroup of patients with HFrEF whoare in AF. 177 However, since this is a retrospective subgroupanalysis, and because beta-blockers did not increase the risk,the guideline committee decided not to make a separate recom-mendation according to heart rhythm. Beta–blockers should beconsidered for rate control in patients with HFrEF and AF, es–pecially in those with high heart rate (see Section 10.1 fordetails).Beta-blockers are recommended in patients with a history ofmyocardial infarction and asymptomatic LV systolicdysfunction to reduce the risk of death (see Section 6).Practical guidance on how to use beta-blockers is given in WebTable 7.5.
7.2.3 Mineralocorticoid/aldosterone receptor antagonists
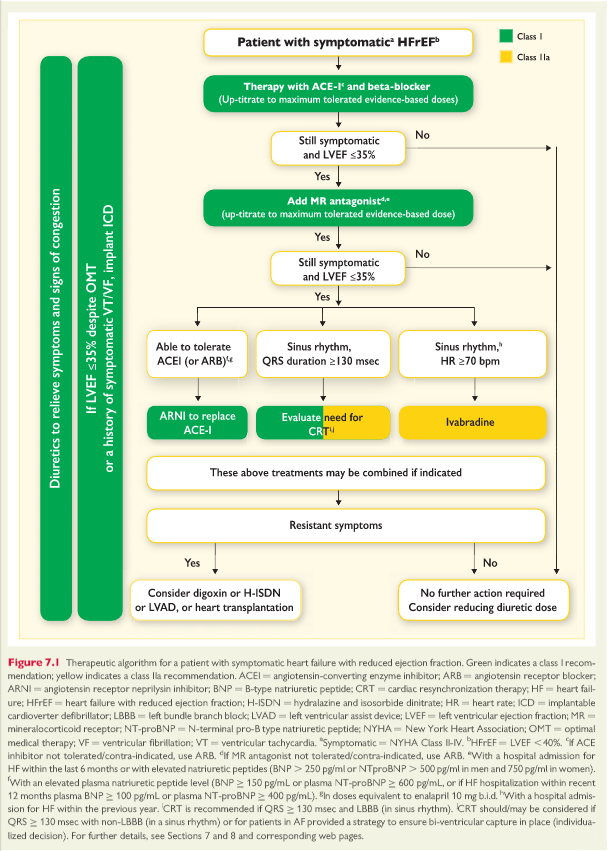
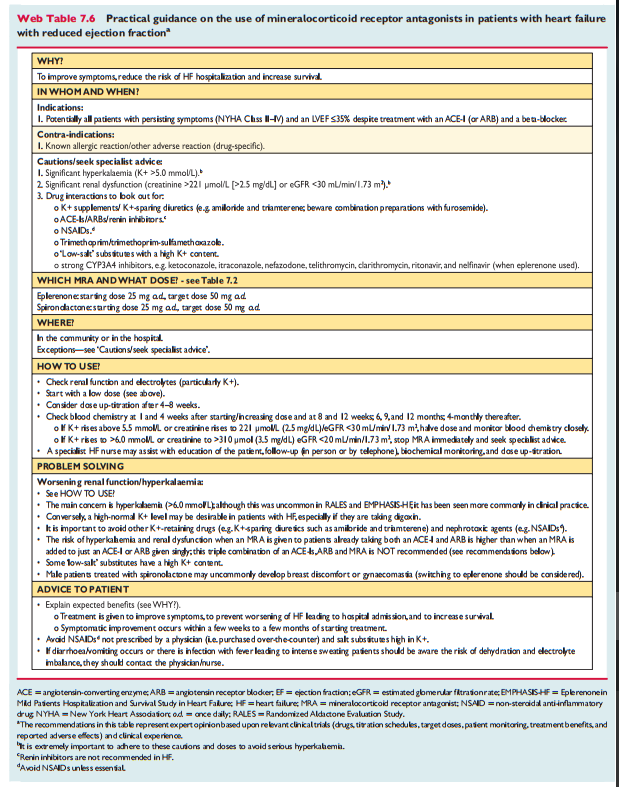
MRAs (spironolactone and eplerenone) block receptors that bind aldosterone and, with different degrees of affinity, other steroid hormone (e.g. corticosteroids, androgens) receptors. Spironolactone or eplerenone are recommended in all symptomatic patients (despite treatment with an ACEI and a beta-blocker) with HFrEF and LVEF ≤35%, to reduce mortality and HF hospitalization.[174, 175]
Caution should be exercised when MRAs are used in patients with impaired renal function and in those with serum potassium levels >5.0 mmol/L. Regular checks of serum potassium levels and renal function should be performed according to clinical status.
Practical guidance on how to use MRAs is given in Web Table 7.6.
7.3 Other treatments recommended in selected symptomatic patients with heart failure with reduced ejection fraction
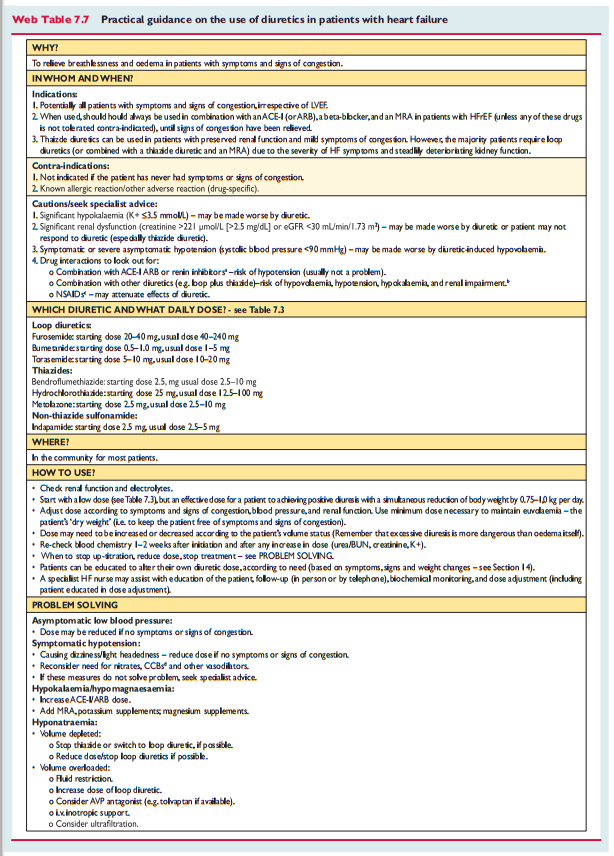
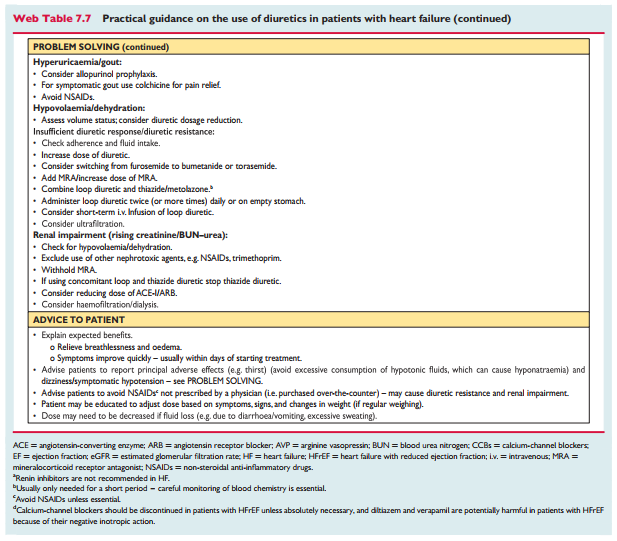
7.3.1 Diuretics
Diuretics are recommended to reduce the signs and symptoms of congestion in patients with HFrEF, but their effects on mortality and morbidity have not been studied in RCTs. A Cochrane meta-analysis has shown that in patients with chronic HF, loop and thiazide diuretics appear to reduce the risk of death and worsening HF compared with placebo, and compared with an active control, diuretics appear to improve exercise capacity.[178, 179]
Loop diuretics produce a more intense and shorter diuresis than thiazides, although they act synergistically and the combination may be used to treat resistant oedema. However, adverse effects are more likely and these combinations should only be used with care. The aim of diuretic therapy is to achieve and maintain euvolaemia with the lowest achievable dose. The dose of the diuretic must be adjusted according to the individual needs over time. In selected asymptomatic euvolaemic/hypovolaemic patients, the use of a diuretic drug might be (temporarily) discontinued. Patients can be trained to self-adjust their diuretic dose based on monitoring of symptoms/signs of congestion and daily weight measurements.
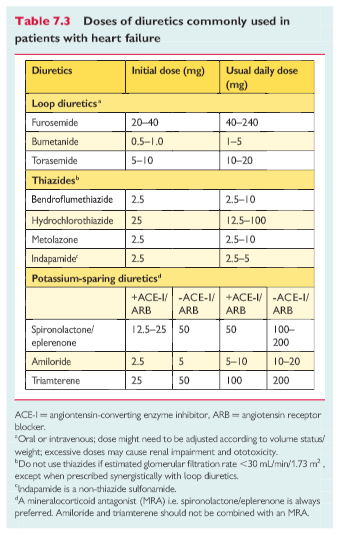
Doses of diuretics commonly used to treat HF are provided in Table7.3. Practical guidance on how to use diuretics is given in Web Table 7.7.
For details on the use of the medicines discussed in the following table please see:
7.3.2 Angiotensin receptor neprilysin inhibitor (pp 913 + 914 of the epdf)
A new therapeutic class of agents acting on the RAAS and the neutral endopeptidase system has been developed [angiotensin receptor neprilysin inhibitor (ARNI)]. The first in class is LCZ696, which is a molecule that combines the moieties of valsartan and sacubitril (neprilysin inhibitor) in a single substance. By inhibiting neprilysin, the degradation of NPs, bradykinin and other peptides is slowed. High circulating A-type natriuretic peptide (ANP) and BNP exert physiologic effects through binding to NP receptors and the aug– mented generation of cGMP, thereby enhancing diuresis, natriuresis and myocardial relaxation and anti-remodelling. ANP and BNP also inhibit renin and aldosterone secretion. Selective AT1-receptorblockade reduces vasoconstriction, sodium and water retention and myocardial hypertrophy. 187,18
[Based on the PARADIGM-HF trial] In this population, sacubitril/valsartan (97/103 mg b.i.d.)was superiortoACEI (enalapril10mgb.i.d.) in reducing hospitalizations for worsening HF, cardiovascular mortality and overall mortality. 162 Sacubitril/valsartan is therefore recommended in patients with HFrEF who fit this profile [meaning the inclusion criteria of the PARADIGM-HF trial].
However, the document notes that there are safety concerns about this medicine related to hypotension and angioedema. However, the most worrisome issue to me is:
There are additional concerns about its effects on the degradation of beta-amyloid peptide in the brain, which could theoretically accelerate amyloid deposition.189 – 191 However, a recent small14-day study with healthy subjects showed elevation of the beta-amyloid protein in the soluble rather than the aggregable form, which if confirmed over longer time periods in patientswith HFrEF may indicate the cerebral safety of sacubitril/valsartan.192 Long-term safety needs to be addressed.
The above concern is very worrisome to me. And that concern is not addressed in the document in terms of the risk reduction benefit of sacubitril/valsartan.
2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure states:
the ARNI [meaning sacubitril/valsartan] reduced the composite endpoint of cardiovascular death or HF hospitalization significantly, by 20% (138). The benefit was seen to a similar extent for both death and HF hospitalization and was consistent across subgroups. The use of ARNI is associated with the risk of hypotension and renal insufficiency and may lead to angioedema, as well.
Returning now to the ESC guidelines:
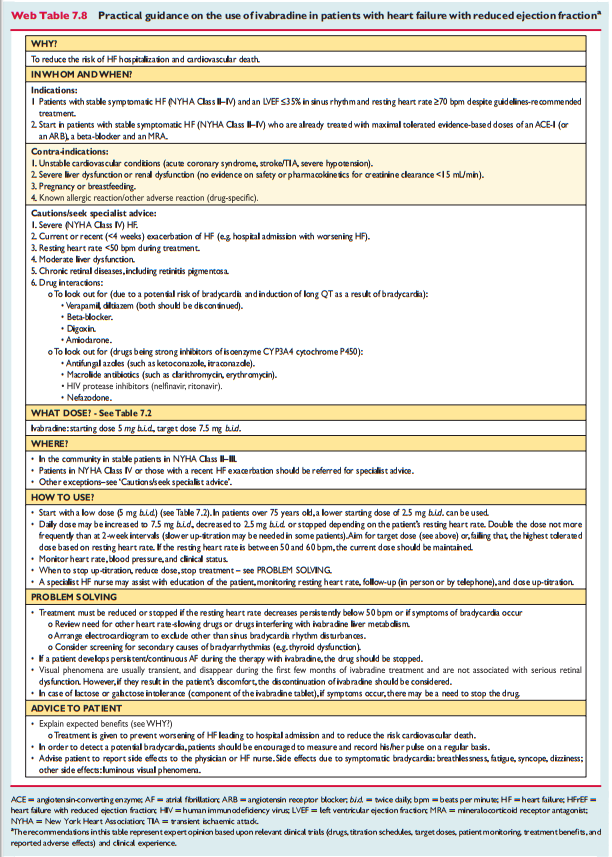
7.3.3 If-channel inhibitor (p 914 of the epdf)
Ivabradine slows the heart rate through inhibition of the Ifchannel in the sinus node and therefore should only be usedfor patients in sinus rhythm. Ivabradine reduced the combinedendpoint of mortality or hospitalization for HF in patients withsymptomatic HFrEF or LVEF ≤35%, in sinus rhythm and with aheart rate ≥70 beats per minute (bpm) who had been hospi-talized for HF within the previous 12 months, receiving treat-ment with an evidence-based dose of beta-blocker (ormaximum tolerated dose), an ACEI (or ARB) and an MRA.180 The European Medicines Agency (EMA) approved ivabradine for use in Europe in patients with HFrEF with LVEF ≤35% and in sinus rhythm with a resting heart rate ≥75 bpm, because in this group ivabradine conferred a survival benefit193 based on a retrospective subgroup analysis requested by the EMA.Practical guidance on how to use ivabradine is given in WebTable 7.8. [Below]
7.3.4 Angiotensin II type I receptor blockers
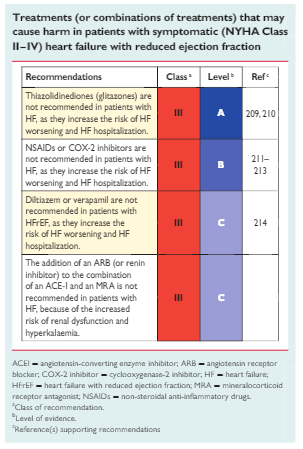
ARBs are recommended only as an alternative in patients intolerant of an ACEI. 182 Candesartan has been shown to reduce cardiovascu-lar mortality. 182Valsartan showed an effect on hospitalization for HF(but not on all-cause hospitalizations) in patients with HFrEF receiv-ing background ACEIs.194The combination of ACEI/ARB for HFrEF was reviewed by theEMA, which suggested that benefits are thought to outweigh risks only in a select group of patients with HFrEF in whom other treat-ents are unsuitable. Therefore, ARBs are indicated for the treatment of HFrEF only in patients who cannot tolerate an ACEI because of serious side effects. The combination of ACEI/ARB should be restricted to symptomatic HFrEF patients receiving a beta-blocker who are unable to tolerate an MRA, and must be used under strict supervision.5.2 Oral anticoagulants and antiplatelet therapyOther than in patients with AF (both HFrEF and HFpEF), there is no evidence that an oral anticoagulant reduces mortality/morbidity compared with placebo or aspirin.206,207 Studies testing the non–vitamin K antagonist oral anticoagulants (NOACs) in patients with HFrEF are currently ongoing. Patients with HFrEF receiving oral anti-coagulation because of concurrent AF or risk of venous thrombo-embolism should continue anticoagulation. Detailed information isprovided in Section 10.1.Similarly, there is no evidence on the benefits of antiplatelet drugs (including acetylsalicylic acid) in patients with HF without accompanying CAD, whereas there is a substantial risk of gastro-intestinal bleeding, particularly in elderly subjects, related with this treatment.
Resources
2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. [PubMed Citation] [Full Text HTML] [Full Text PDF]. Eur J Heart Fail. 2016 Aug;18(8):891-975. doi: 10.1002/ejhf.592. Epub 2016 May 20.
2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart
failure – Web Addenda [Full Text PDF -you may need t register for a free ESC account to access this resource]