Today, I review, link to, and excerpt from my Google Search, high-resolution coronary computed tomography angiography (CCTA) with artificial intelligence. Search performed on 8-20-2025.
All that follows is from the above resource.
AI Overview
High-resolution coronary computed tomography angiography (CCTA) with artificial intelligence (AI)-guided plaque quantification is a powerful tool for assessing coronary artery disease (CAD). It combines the detailed anatomical information from CCTA with AI algorithms to automatically analyze and quantify plaque characteristics, offering a more precise and potentially personalized approach to risk assessment and treatment planning.
Here’s a breakdown of the key aspects:
CCTA provides high-resolution images of the coronary arteries, allowing visualization of plaque buildup and potential narrowing (stenosis).
AI algorithms analyze these images to automatically quantify various plaque characteristics, such as:
- Total Plaque Volume (TPV):The overall amount of plaque in the coronary arteries.
- Non-calcified Plaque (NCP):Plaque that is primarily composed of fat and other substances, often considered more vulnerable to rupture.
- Calcified Plaque (CP):Plaque containing calcium, generally associated with lower risk.
- High-Risk Plaque (HRP) features:Specific plaque characteristics, like low attenuation or spotty calcifications, that may indicate a higher risk of future events.
This approach offers several advantages:
- Improved Diagnostic Precision:AI can identify subtle plaque features that might be missed by human readers, leading to a more accurate assessment of CAD.
- Refined Risk Stratification:By quantifying plaque burden and characteristics, AI-QCT can help better identify patients at higher risk of future cardiac events.
- Personalized Treatment:Quantifying plaque components can guide decisions about lipid-lowering therapy and other interventions to stabilize or reduce plaque burden.
- Increased Efficiency:AI-QCT can automate some of the more time-consuming aspects of CCTA analysis, potentially streamlining the diagnostic process.
- Potential for Improved Outcomes:By identifying high-risk plaques and guiding treatment decisions, AI-QCT has the potential to improve patient outcomes and reduce the risk of heart attacks and other cardiovascular events.
AI-QCT is being used in several clinical settings:
- Assessing risk in patients with suspected CAD:CMS (.gov) says CCTA with AI-QCT is now considered a first-line tool in evaluating acute and chronic CAD, particularly in symptomatic patients with intermediate or high pre-test probability of obstructive CAD.
- Guiding treatment decisions:AI-QCT can help determine the most appropriate treatment strategy, including whether to initiate or adjust lipid-lowering therapy.
- Monitoring disease progression and treatment response:AI-QCT can be used to track changes in plaque burden and composition over time, allowing for assessment of treatment effectiveness.
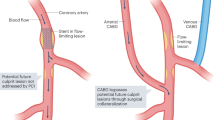
- Improving pre-procedural planning for coronary revascularization:JSCAI says CCTA with AI-QCT can help determine the best approach for procedures like angioplasty or bypass surgery.
In essence, AI-guided plaque quantification in CCTA is revolutionizing the way we assess and manage coronary artery disease, offering a more precise, efficient, and personalized approach to patient care.
Here are the websites that this Google Search linked to.
Coronary Computed Tomography Angiography to Guide Percutaneous Coronary Intervention: Expert Opinion from a SCAI/SCCT Roundtable. Volume 4, Issue 6103664June 2025
Abstract
Coronary computed tomography angiography (CCTA) has emerged as an important tool for planning percutaneous coronary intervention (PCI). While it has traditionally been employed for diagnostic purposes, increasing evidence and real-world experience suggest that CCTA can be used for the preprocedural planning of PCI and can inform patient triage, shared decision making, case complexity, and resource use. This approach mirrors how computed tomography angiography is routinely used to plan structural interventions. To address these emerging opportunities, the Society for Cardiovascular Angiography & Interventions (SCAI) and the Society of Cardiovascular Computed Tomography (SCCT) organized a multidisciplinary, expert scientific roundtable on the use of CCTA for guiding PCI. The goal of this document is to provide a state-of-the-art overview of CCTA-guided PCI focused on practical applications and key coronary lesion subsets, define unmet needs and barriers, and outline future directions.Issue Description
This policy was developed based on a Local Coverage Determination (LCD) request for coverage for Quantitative Coronary Plaque Analysis (QCPA) using Artificial Intelligence Enabled CT Based Quantitative Coronary Topography (AI-QCT)/Coronary Plaque Analysis (AI-CPA). Specific details outlining coverage for this service is located under the Coverage Indications, Limitations and/or Medical Necessity section of this LCD.Coverage Guidance
Coverage Indications, Limitations, and/or Medical Necessity
Artificial Intelligence Enabled CT Based Quantitative Coronary Topography/Coronary Plaque Analysis (AI-QCT/AI-CPA) using coronary computed tomography angiography (CCTA)* is considered reasonable and medically necessary as a diagnostic study when:The patient has acute or stable chest pain with no known coronary artery disease (CAD)1 and is eligible for CCTA*, AND
CCTA classifies patient as:
Intermediate risk** OR
CAD-RADS 1, CAD-RADS 2 or CAD-RADS 3 category*** on CCTA1,2, AND
Cardiac evaluation is negative or inconclusive for acute coronary syndrome (ACS)1.
*See Local Coverage Determination (LCD), Cardiac Computed Tomography & Angiography (CCTA) L33423 for criteria for CCTA.AI-QCT/AI-CPA should not be performed until after the base study (CCTA) has been completed and interpreted. Software to perform AI-QCT/AI-CPA must be FDA cleared or approved.
**Intermediate and high-risk as defined in the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain1
*** CAD-RADS 1- CAD-RADS 3 category as defined by CAD-RADS™ 2.0–2022 Coronary Artery Disease Reporting and Data System (CAD-RADS): an Expert Consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI).2,3
Limitations
AI-QCT/AI-CPA is NOT considered reasonable or necessary in the following clinical circumstance(s) (non-covered):
Screening (i.e., in the absence of signs, symptoms, or disease);
When there are contraindications to CCTA*;
In conjunction with invasive coronary catheterization;
In the presence of normal CCTA results (CAD RADS=0 or no plaque disease);
In the presence of high-grade stenosis (>70%) or CAD-RADS 4 and CAD-RADS 5;
Within 30 days of a myocardial infarction (MI);
In the presence of unstable coronary symptoms; and
For disease surveillance.
*See Cardiac Computed Tomography & Angiography (CCTA) L33423 for a full listing of contraindications to CCTA.AI-Guided Quantitative Plaque Staging Predicts Long-Term Cardiovascular Outcomes in Patients at Risk for Atherosclerotic CVD. JACC: Cardiovascular Imaging
Volume 17, Number 3Abstract
Background
The recent development of artificial intelligence–guided quantitative coronary computed tomography angiography analysis (AI-QCT) has enabled rapid analysis of atherosclerotic plaque burden and characteristics.
Objectives
This study set out to investigate the 10-year prognostic value of atherosclerotic burden derived from AI-QCT and to compare the spectrum of plaque to manually assessed coronary computed tomography angiography (CCTA), coronary artery calcium scoring (CACS), and clinical risk characteristics.
Methods
This was a long-term follow-up study of 536 patients referred for suspected coronary artery disease. CCTA scans were analyzed with AI-QCT and plaque burden was classified with a plaque staging system (stage 0: 0% percentage atheroma volume [PAV]; stage 1: >0%-5% PAV; stage 2: >5%-15% PAV; stage 3: >15% PAV). The primary major adverse cardiac event (MACE) outcome was a composite of nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and all-cause mortality.
Results
The mean age at baseline was 58.6 years and 297 patients (55%) were male. During a median follow-up of 10.3 years (IQR: 8.6-11.5 years), 114 patients (21%) experienced the primary outcome. Compared to stages 0 and 1, patients with stage 3 PAV and percentage of noncalcified plaque volume of >7.5% had a more than 3-fold (adjusted HR: 3.57; 95% CI 2.12-6.00; P < 0.001) and 4-fold (adjusted HR: 4.37; 95% CI: 2.51-7.62; P < 0.001) increased risk of MACE, respectively. Addition of AI-QCT improved a model with clinical risk factors and CACS at different time points during follow-up (10-year AUC: 0.82 [95% CI: 0.78-0.87] vs 0.73 [95% CI: 0.68-0.79]; P < 0.001; net reclassification improvement: 0.21 [95% CI: 0.09-0.38]). Furthermore, AI-QCT achieved an improved area under the curve compared to Coronary Artery Disease Reporting and Data System 2.0 (10-year AUC: 0.78; 95% CI: 0.73-0.83; P = 0.023) and manual QCT (10-year AUC: 0.78; 95% CI: 0.73-0.83; P = 0.040), although net reclassification improvement was modest (0.09 [95% CI: −0.02 to 0.29] and 0.04 [95% CI: −0.05 to 0.27], respectively).
Conclusions
Through 10-year follow-up, AI-QCT plaque staging showed important prognostic value for MACE and showed additional discriminatory value over clinical risk factors, CACS, and manual guideline-recommended CCTA assessment.
Atherosclerotic cardiovascular disease (ASCVD) risk stratification using traditional clinical risk factors may overestimate ASCVD events and current guideline-recommended 10-year risk stratification performs relatively poorly in identifying patients at high risk for ASCVD.1-4 Coronary computed tomography angiography (CCTA) permits direct visualization of coronary artery disease (CAD) with high diagnostic performance and unique evaluation of atherosclerosis that allows for enhanced risk prognostication.Artificial Intelligence-Based Quantitative Coronary Plaque Analysis
Editorial Comment:13 September 2023
Beyond depicting the coronary lumen and facilitating assessment of stenosis, coronary computed tomography angiography (CCTA) images the presence, morphology (composition), and severity of coronary plaque.1 Over the past decade this unique ability has been extensively leveraged, with numerous studies consistently showing that CCTA-based coronary plaque assessments have important clinical implications. These scientific efforts have initially been focused on qualitative analysis of adverse plaque features, including the low attenuation plaque, positive remodeling, and spotty calcifications that have been perceived as the quintessential high-risk or vulnerable plaque.2,3
Notwithstanding the initial enthusiasm, the positive predictive value of adverse plaque features in distinguishing patients who may experience future myocardial infarction remains limited.2,3 Moreover, the predictive value of adverse plaque features was not independent of affordable disease burden assessments, such as the coronary artery calcium score.3 These limitations, along with the paradigm that the risk of adverse coronary events should be perceived on a per-patient rather than a per-lesion basis, have led to a growing interest in quantifying plaque volumes.4,5 To this end, several software packages for quantitative plaque analysis have emerged, enabling detailed characterization of the composition, volume, and burden of atherosclerotic plaque on the patient level.5,6
In summary, AI has the potential to facilitate the transition of quantitative plaque analysis from research to a powerful clinical application. This important technological progress complements the emerging shift from a focus on luminal stenosis to a more “plaque-centered” approach, which considers the burden, composition, and activity of the atherosclerotic lesions.7,13-16
Coronary CT angiography evaluation with artificial intelligence for individualized medical treatment of atherosclerosis: a Consensus Statement from the QCI Study Group. Published: 01 August 2025. Nature Reviews Cardiology (2025).
Abstract
Coronary CT angiography is widely implemented, with an estimated 2.2 million procedures in patients with stable chest pain every year in Europe alone. In parallel, artificial intelligence and machine learning are poised to transform coronary atherosclerotic plaque evaluation by improving reliability and speed. However, little is known about how to use coronary atherosclerosis imaging biomarkers to individualize recommendations for medical treatment. This Consensus Statement from the Quantitative Cardiovascular Imaging (QCI) Study Group outlines key recommendations derived from a three-step Delphi process that took place after the third international QCI Study Group meeting in September 2024. Experts from various fields of cardiovascular imaging agreed on the use of age-adjusted and gender-adjusted percentile curves, based on coronary plaque data from the DISCHARGE and SCOT-HEART trials. Two key issues were addressed: the need to harness the reliability and precision of artificial intelligence and machine learning tools and to tailor treatment on the basis of individualized plaque analysis. The QCI Study Group recommends that the presence of any atherosclerotic plaque should lead to a recommendation of pharmacological treatment, whereas the 70th percentile of total plaque volume warrants high-intensity treatment. The aim of these recommendations is to lay the groundwork for future trials and to unlock the potential of coronary CT angiography to improve patient outcomes globally.
Similar content being viewed by others
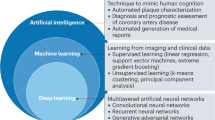
Artificial intelligence in coronary computed tomography angiography. Medicine Plus. Volume 1, Issue 1, March 2024, 100001Abstract
The rapid development of artificial intelligence (AI) technologies, like machine learning, deep learning, and other algorithms applied to the intelligent diagnostic and decision making, image interpretation, accurate classification, and prognostication of cardiovascular diseases, has led to broad application prospects and innovation potential. The digital and intelligent management model of cardiovascular disease is expected to improve the management level and efficiency of diseases and provide patients with more accurate, safe, and appropriate diagnosis and treatment methods. This review systematically introduces the common AI techniques in the field of cardiovascular computed tomography (CT), summarizes the current research and application progress of AI in cardiovascular CT, and provides its future perspectives.Enhancing coronary artery plaque analysis via artificial intelligence-driven cardiovascular computed tomography. Ther Adv Cardiovasc Dis. 2024 Dec 3;18:17539447241303399. doi: 10.1177/17539447241303399Abstract
Coronary computed tomography angiography (CCTA) is a noninvasive imaging modality of cardiac structures and vasculature considered comparable to invasive coronary angiography for the evaluation of coronary artery disease (CAD) in several major cardiovascular guidelines. Conventional image acquisition, processing, and analysis of CCTA imaging have progressed significantly in the past decade through advances in technology, computation, and engineering. However, the advent of artificial intelligence (AI)-driven analysis of CCTA further drives past the limitations of conventional CCTA, allowing for greater achievements in speed, consistency, accuracy, and safety. AI-driven CCTA (AI-CCTA) has achieved a significant reduction in radiation exposure for patients, allowing for high-quality scans with sub-millisievert radiation doses. AI-CCTA has demonstrated comparable accuracy and consistency in manual coronary artery calcium scoring against expert human readers. An advantage over invasive coronary angiography, which provides luminal information only, CCTA allows for plaque characterization, providing detailed information on the quality of plaque and offering further prognosticative value for the management of CAD. Combined with AI, many recent studies demonstrate the efficacy, accuracy, efficiency, and precision of AI-driven analysis of CCTA imaging for the evaluation of CAD, including assessing degree stenosis, adverse plaque characteristics, and CT fractional flow reserve. The limitations of AI-CCTA include its early phase in investigation, the need for further improvements in AI modeling, possible medicolegal implications, and the need for further large-scale validation studies. Despite these limitations, AI-CCTA represents an important opportunity for improving cardiovascular care in an increasingly advanced and data-driven world of modern medicine.
Keywords: APC, artificial intelligence, CAC, CAD, CCTA, CONFIRM, DL, FFT-CT, ML Artificial intelligence in coronary computed tomography angiography: Demands and solutions from a clinical perspective. Front. Cardiovasc. Med., 15 February 2023. Sec. Cardiovascular Imaging. Volume 10 – 2023 | https://doi.org/10.3389/fcvm.2023.1120361
Coronary computed tomography angiography (CCTA) is increasingly the cornerstone in the management of patients with chronic coronary syndromes. This fact is reflected by current guidelines, which show a fundamental shift towards non-invasive imaging – especially CCTA. The guidelines for acute and stable coronary artery disease (CAD) of the European Society of Cardiology from 2019 and 2020 emphasize this shift. However, to fulfill this new role, a broader availability in adjunct with increased robustness of data acquisition and speed of data reporting of CCTA is needed. Artificial intelligence (AI) has made enormous progress for all imaging methodologies concerning (semi)-automatic tools for data acquisition and data post-processing, with outreach toward decision support systems. Besides onco- and neuroimaging, cardiac imaging is one of the main areas of application. Most current AI developments in the scenario of cardiac imaging are related to data postprocessing. However, AI applications (including radiomics) for CCTA also should enclose data acquisition (especially the fact of dose reduction) and data interpretation (presence and extent of CAD). The main effort will be to integrate these AI-driven processes into the clinical workflow, and to combine imaging data/results with further clinical data, thus – beyond the diagnosis of CAD- enabling prediction and forecast of morbidity and mortality. Furthermore, data fusing for therapy planning (e.g., invasive angiography/TAVI planning) will be warranted. The aim of this review is to present a holistic overview of AI applications in CCTA (including radiomics) under the umbrella of clinical workflows and clinical decision-making. The review first summarizes and analyzes applications for the main role of CCTA, i.e., to non-invasively rule out stable coronary artery disease. In the second step, AI applications for additional diagnostic purposes, i.e., to improve diagnostic power (CAC = coronary artery classifications), improve differential diagnosis (CT-FFR and CT perfusion), and finally improve prognosis (again CAC plus epi- and pericardial fat analysis) are reviewed.
A Convolutional Neural Network for Automatic Characterization of Plaque Composition in Carotid Ultrasound. IEEE J Biomed Health Inform. 2016 Nov 22;21(1):48–55. doi: 10.1109/JBHI.2016.2631401
Abstract
Characterization of carotid plaque composition, more specifically the amount of lipid core, fibrous tissue, and calcified tissue, is an important task for the identification of plaques that are prone to rupture, and thus for early risk estimation of cardiovascular and cerebrovascular events. Due to its low costs and wide availability, carotid ultrasound has the potential to become the modality of choice for plaque characterization in clinical practice. However, its significant image noise, coupled with the small size of the plaques and their complex appearance, makes it difficult for automated techniques to discriminate between the different plaque constituents. In this paper, we propose to address this challenging problem by exploiting the unique capabilities of the emerging deep learning framework. More specifically, and unlike existing works which require a priori definition of specific imaging features or thresholding values, we propose to build a convolutional neural network that will automatically extract from the images the information that is optimal for the identification of the different plaque constituents. We used approximately 90,000 patches extracted from a database of images and corresponding expert plaque characterizations to train and to validate the proposed convolutional neural network (CNN). The results of cross-validation experiments show a correlation of about 0.90 with the clinical assessment for the estimation of lipid core, fibrous cap, and calcified tissue areas, indicating the potential of deep learning for the challenging task of automatic characterization of plaque composition in carotid ultrasound
Index Terms: Atherosclerosis, carotid artery, ultrasound, plaque composition, convolutional neural networks
Artificial Intelligence in Coronary Artery Interventions: Preprocedural Planning and Procedural Assistance. JSCIA. Volume 4, Issue 3102519March 2025
Keywords
Abbreviations
- 3D (3-dimensional)
- AI (artificial intelligence)
- CABG (coronary artery bypass graft)
- CCTA (cardiac computed tomography angiography)
- CMR (cardiac magnetic resonance)
- FFR (fractional flow reserve)
- ICA (invasive coronary angiography)
- IVUS (intravascular ultrasound)
- OCT (optical coherence tomography)
- PCI (percutaneous coronary artery intervention)