Today, I review, link to, and embed EM Note’s Euglycemic DKA.
All that follows is from the above resource.
Homepage: EMNote.org ■
🚩Membership: https://tinyurl.com/joinemnote
🚩ACLS Lecture: https://tinyurl.com/emnoteacls
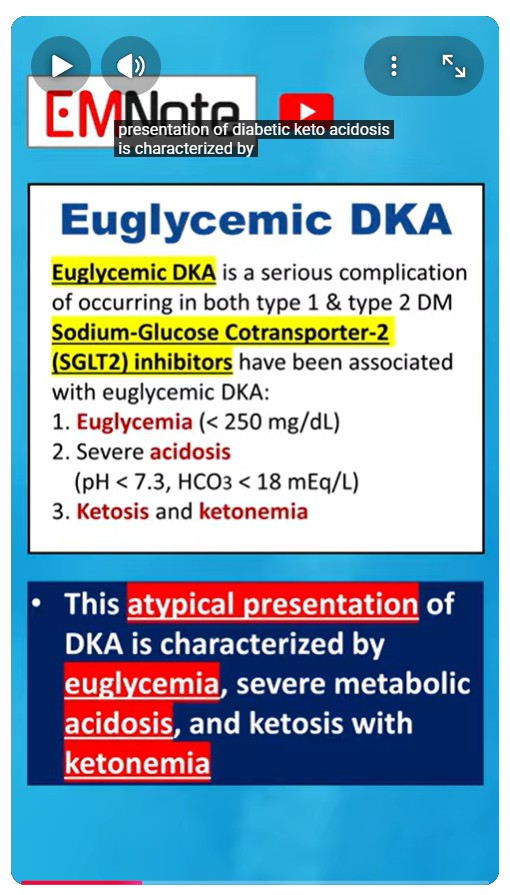
Euglycemic diabetic ketoacidosis (EDKA) is a critical condition that can affect individuals with both type 1 and type 2 diabetes mellitus. This atypical presentation of diabetic ketoacidosis is characterized by euglycemia (blood glucose less than 250 mg/dL), severe metabolic acidosis (pH less than 7.3, bicarbonate less than 18 mEq/L), and ketosis with ketonemia. Sodium-Glucose Cotransporter-2 (SGLT2) inhibitors, a class of antidiabetic medications, have been implicated in the development of EDKA. These agents function by inhibiting glucose reabsorption in the proximal renal tubules, thereby promoting urinary glucose excretion and reducing blood glucose levels. The pathophysiology of SGLT2 inhibitor-associated EDKA involves decreased insulin production, increased glucagon secretion, and a metabolic shift favoring lipolysis and ketogenesis.