Today’s article is based on 2024 Guideline for the Primary Prevention of Stroke: A Guideline From the American Heart Association/American Stroke Association [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Stroke
. 2024 Dec;55(12):e344-e424. doi: 10.1161/STR.0000000000000475. Epub 2024 Oct 21.
All that follows is from Future of Stroke Prevention: 7 Updates in the 2024 AHA/ASA Primary Prevention of Stroke Guideline. JACC: Advances
Volume 4, Number 6_Part_2
Abstract
Approximately 9 to 10 million adults (4%) have experienced a stroke in the United States. While stroke incidence has generally declined, progress has been less pronounced among young individuals, and such trends have underlined the importance of focusing on the primary prevention of stroke. In 2024, the American Heart Association and American Stroke Association released new guidelines for the primary prevention of stroke. Here, we review major updates in 7 domains: dietary modification, glucagon-like peptide-1 receptor agonists, blood pressure targets, lipid-lowering medications, antithrombotic agents, colchicine therapy, and sex-specific preventive risk assessment. Through this process, we review important randomized controlled trial evidence contributing to guideline updates and provide key perspectives on the incorporation of lifestyle and pharmacotherapy for personalized stroke prevention.Highlights
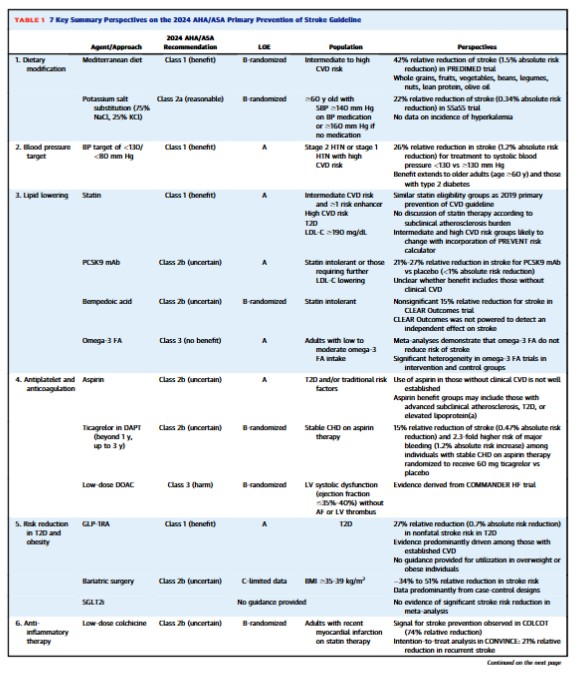
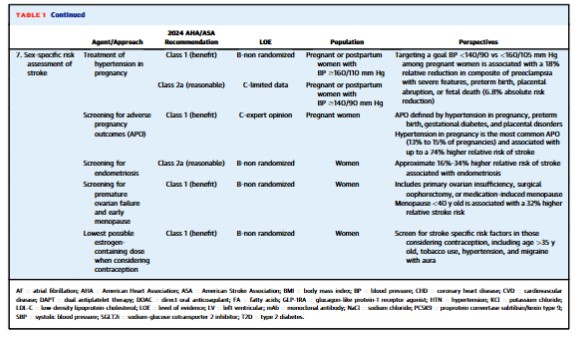
• Declines in stroke incidence have been less pronounced in younger adults.• Benefits of <130/<80 mm Hg blood pressure extend to diabetes and older adults.• New Class 1 indication for GLP-1RA in type 2 diabetes for stroke risk reduction.• Further research required to define the precise role of colchicine in stroke risk reduction.The prevalence of stroke in the United States is approximately 4% (9-10 million individuals), approaching 7% in persons ≥60 years old and 14% in those ≥80 years old.1 By 2050, the prevalence of stroke will likely have increased by nearly 66% when compared to 2020.2 While the incidence of stroke is generally declining in high-income countries,3 such progress is less pronounced among younger individuals—with stroke incidence rates remaining relatively flat or even increasing among those below age <55 years.4,5 Such trends underline the importance of focusing on the primary prevention of stroke among all age groups. Here we review 7 key updates to the recent6 American Heart Association/American Stroke Association 2024 Guideline on the Primary Prevention of Stroke.6This 2024 Guideline embraces Life’s Essential 8 for the promotion of cardiovascular disease (CVD) and brain health across the life course.7 In addition to emphasizing the importance of routine primary care and addressing adverse social determinants of health, this guideline featured major updates in 7 key domains for personalized stroke prevention: dietary modification, glucagon-like peptide-1 receptor agonists (GLP-1RA), blood pressure (BP) targets, lipid-lowering medications, antithrombotic agents, colchicine therapy, and sex-specific preventive considerations. In this review, we highlight advances in these key domains and the latest practice-changing literature behind the guidelines (Table 1, Central Illustration).Dietary modificationThe latest guidelines underscore the preventive utility of the Mediterranean diet and salt substitution. With respect to nonpharmacological approaches for incident stroke risk reduction, the notable updates in the 2024 Guideline include a higher (Class 1, strong; indicating benefits substantially outweigh potential harms) recommendation for the Mediterranean diet and formal discussions involving potassium salt substitution. Among individuals with intermediate to high CVD risk, defined by the presence of 2 or more risk factors, a meta-analysis of randomized controlled trials (RCTs) indicates that Mediterranean diet interventions may reduce the risk of stroke by approximately 35% when compared to placebo-controlled diets.8 Importantly, Mediterranean dietary intervention was more strongly associated with stroke risk reduction (HR: 0.58; 95% CI: 0.42-0.82) when compared to myocardial infarction (HR: 0.80; 95% CI: 0.53-1.21) or CVD death (HR: 0.80; 95% CI: 0.51-1.24) in the PREDIMED (Prevention with Mediterranean Diet) trial.9While the DASH (Dietary Approaches to Stop Hypertension) diet had a previous Class 1 indication in the 2014 Guideline due to RCT evidence for systolic BP reduction,10 no specific recommendations involving DASH were made in the 2024 update given the lack of RCT evidence including incident stroke as an outcome. Nevertheless, observational evidence suggests that each 4-point increase in DASH diet score is associated with a 4% relative risk reduction in stroke.11 Both the DASH and Mediterranean diets emphasize whole grains, fruits, and vegetables, though the Mediterranean diet has a higher average percentage of calories from monounsaturated (eg, olive oil) and polyunsaturated fat (eg, salmon, walnuts). In contrast to the Mediterranean and DASH diets, there is limited evidence that low fat dietary interventions result in significant risk reductions for stroke. Beyond the Mediterranean and DASH diets, observational evidence from the Nurses’ Health Study suggests that adherence to a healthy plant-based diet may be associated with an 8% lower risk of ischemic stroke.12The 2024 Guideline on the Primary Prevention of Stroke provided a new Class 2a (moderate; indicating benefits likely outweigh potential harms) recommendation for the use of salt substitutes (75% sodium chloride, 25% potassium chloride) rather than table salt (100% sodium chloride) to reduce stroke risk among adults ≥60 years of age with uncontrolled hypertension (systolic BP ≥140 mm Hg without medication, ≥160 on medication). This recommendation is based largely on a 22% relative risk reduction (1.17% vs 1.51%) in incident stroke among more than 5,000 individuals without prevalent stroke within a recent open-label, cluster RCT conducted in rural China.13 Additionally, results for the entire trial (individuals with and without stroke at baseline included) also demonstrated a significant 14% relative risk reduction in stroke with potassium salt substitution over 4.7 years. Notably, serum potassium levels were not measured in the primary RCT conducted involving salt substitution and clinicians should monitor closely for incident hyperkalemia among individuals on potassium-sparing diuretics, potassium supplementation, and/or those with chronic kidney disease (CKD).7GLP-1RA
GLP-1RA, such as semaglutide and dulaglutide, are recognized as a new class of medications for primary stroke prevention. Since 2019, utilization of GLP-1RA has increased by 7-fold in the United States.14 The 2024 Guideline provides a Class 1 (strong) recommendation for the utilization of GLP-1RA among individuals with a high predicted CVD risk or established CVD (including stroke) with a glycated hemoglobin value of ≥7% for primary and secondary stroke prevention. Meta-analyses of RCTs including GLP-1RA therapy among individuals with diabetes have indicated an approximate 27% reduction in nonfatal stroke risk—particularly driven by protection from ischemic stroke.15Most individuals in RCTs involving GLP-1RA in type 2 diabetes have had prevalent CVD (73%-85%).15 The GLP-1RA outcomes trial in type 2 diabetes that included the most individuals without clinical CVD (∼70%) was REWIND (Research Cardiovascular Events with a Weekly Incretin in Diabetes).16 In the REWIND trial, <7% of participants had prevalent stroke at baseline and dulaglutide was associated with a 24% relative reduction in nonfatal stroke compared to placebo over a median 5.4-period follow-up (3.2% vs 4.1%; HR: 0.76; 95% CI: 0.62-0.94). There was no significant interaction for the protective association between dulaglutide and CVD according to baseline presence or absence of baseline clinical CVD.16 Further research is required to elucidate whether GLP-1RA therapy in those without type 2 diabetes with overweight or obesity reduces the risk of incident or recurrent stroke.16 Beyond GLP-1RA, meta-analysis of RCTs has not found that sodium-glucose cotransporter-2 inhibitors therapy significantly reduces the risk of stroke among individuals who have type 2 diabetes with or without clinical CVD.17BP targets
Similar to recent major societal guidelines,18,19 the 2024 Guideline for the Primary Prevention of Stroke provides a Class 1 (strong) recommendation for targeting a new (lower compared to the 2014 stroke guideline) systolic BP target of <130 and diastolic BP <80 mm Hg in adults at higher risk for atherosclerotic CVD.6 While higher risk for atherosclerotic CVD was not formally defined in the 2024 Guideline, it may be inferred based on RCT evidence that this population is generally reflective of individuals with clinical CVD, advanced subclinical atherosclerosis, CKD, type 2 diabetes, older adults, or a high 10-year risk of CVD. Rigorous evidence has demonstrated a benefit of targeting a systolic BP of <130 and diastolic BP <80 mm Hg for the primary and secondary prevention of stroke,20 including older adults ≥60 years of age and those with type 2 diabetes. Each 5 mm Hg reduction in systolic BP reduction confers an approximate 13% lower risk of stroke among individuals with and without clinical CVD.In the STEP (Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients) trial,21 individuals randomized to intensive vs standard antihypertensive treatment (<130 vs <150 mm Hg) experienced a 33% lower risk of incident stroke over 1 year of follow-up (0.3% vs 0.5%). Among patients with diabetes, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) BP trial targeted a systolic BP <120 vs <140 mm Hg and observed a 41% lower risk of stroke over 1 year (0.3% vs 0.5%), which was a secondary outcome of the trial.22 While there was no significant risk reduction observed for the primary outcome of major adverse cardiovascular events in ACCORD, this may have been due to the factorial design (BP and glucose-lowering) of the ACCORD trial.More recently, after the 2024 Guideline’s publication, the BPROAD (Blood Pressure Control Target in Diabetes) trial demonstrated that intensive treatment to a systolic BP of ≤120 vs ≤140 mm Hg among more than 12,000 individuals with type 2 diabetes resulted in a 21% relative risk reduction in stroke (1.65% vs 2.09%) over 5 years.23 Importantly, this reduction in stroke appeared to be largely responsible for the reduction in the primary composite CVD outcome. One meta-analysis has demonstrated a 26% relative reduction in stroke (HR: 0.74; 95% CI: 0.66-0.84; 3.3% vs 4.5%) among 7 RCTs assessing antihypertensive targeting a systolic BP of <130 vs ≥130 mm Hg, and a 19% relative reduction in stroke (HR: 0.81; 95% CI: 0.70-0.94; 3.1% vs 3.7%) among 4 RCTs targeting a systolic BP of <120 vs ≤140 mm Hg.24 Based on RCT evidence, the 2024 European Society of Cardiology Guideline for the Management of Elevated Blood Pressure and Hypertension provide a Class 1 recommendation to target a systolic BP treatment target of 120 to 129 mm Hg if therapy is well-tolerated.25Lipid-lowering therapy
Similar to the 2019 American College of Cardiology/American Heart Association Primary Prevention of CVD Guideline,18 the 2024 Primary Prevention of Stroke Guideline recommends (Class 1) primary prevention statin therapy across 4 statin eligibility groups (low-density lipoprotein-cholesterol [LDL-C] ≥190 mg/dL, type 2 diabetes, 10-year CVD risk ≥20%, and 10-year CVD risk between 7.5% and 19.9% with at least one risk enhancer).18,26 However, the classification for intermediate and high risk may change when guideline updates use the 2023 PREVENT (Predicting Risk of Cardiovascular Disease Events) calculator,27,28 * which generally provides a risk estimate 30% to 50% lower compared to the 2013 Pooled Cohort Equations owing to its derivation in a much larger and more contemporary sample.* See Linking To The AHA’s Predicting Risk of cardiovascular disease EVENTs (PREVENT) Online Calculator
Posted on August 23, 2025 by Tom Wade MDUtilization of proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies (mAbs) for statin-eligible individuals who are statin intolerant or require further LDL-C lowering on maximally tolerated statin therapy was given a 2b recommendation (uncertain benefit). This recommendation is made based on the fact that: 1) no dedicated primary prevention trials involving PCSK9 mAbs (evolocumab, alirocumab) have been performed; and 2) one meta-analysis identified 21% to 27% risk reduction (95% CI: 6%-42%) in stroke for PCSK9 mAbs when compared to placebo but did not specify whether individuals had prevalent stroke at baseline, thereby making recommendations for primary prevention of stroke unclear.29In the ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab) trial, alirocumab conferred a lower risk of fatal and nonfatal stroke in secondary outcome analysis (27% relative risk reduction, 1.2% vs 1.6%) over nearly 3 years among persons with acute coronary syndrome within the last 12 months.30 Approximately 3% of individuals in ODYSSEY OUTCOMES had prevalent stroke and all were on maximally tolerated statin therapy. However, evidence from the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial did not show a significant risk reduction in stroke with evolocumab within secondary outcome analysis.31,32New recommendations for the utilization of bempedoic acid and omega-3 fatty acid supplementation/pharmacotherapy were provided in the 2024 Primary Prevention of Stroke Guideline. Use of bempedoic acid among individuals with statin-associated side effects received a 2b recommendation in the 2024 Primary Prevention of Stroke Guideline. In secondary RCT outcomes, bempedoic acid led to a nonsignificant 15% relative risk reduction (HR: 0.85; 95% CI: 0.67-1.07) in fatal or nonfatal stroke; however, the CLEAR Outcomes trial was not powered to detect an independent effect on stroke.33A Class 3 recommendation (no benefit) was provided for omega-3 fatty acid supplementation for stroke risk reduction. Several different meta-analyses have demonstrated that omega-3 fatty acid supplementation does not reduce risk of stroke.34,35 However, not all RCTs involving omega-3 fatty acids included in these meta-analyses have included similar control groups36,37 or similar omega-3 fatty acid formulations, which may be an important consideration given that high-dose eicosapentaenoic acid and icosapent ethyl monotherapy may have enhanced CVD protective benefit compared to combinations including docosahexaenoic acid.38 REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) found that 2 g of twice daily icosapent ethyl vs mineral oil placebo led to a 28% relative risk reduction in a secondary outcome of stroke (2.4% vs 3.3%),39 although subgroup analyses were not reported according to the presence vs absence of baseline stroke and/or prevalent clinical CVD. Providers should remain focused on optimal statin therapy, with the consideration of PCSK9 mAb for further LDL-C lowering or bempedoic acid in those with statin intolerance.Antiplatelet and anticoagulation therapies
The 2024 Guideline on the Primary Prevention of Stroke emphasizes the judicious and intentional use of antithrombotic and anticoagulant agents for stroke prevention. In contrast to the 2014 Stroke Prevention Guideline that provided a Class 2a recommendation (reasonable) for the utilization of aspirin therapy among individuals without clinical CVD who had a 10-year risk ≥10% or those with diabetes,26 the 2019 Primary Prevention of CVD Guideline18 provided a 2b recommendation (uncertain) for the utilization of aspirin therapy in those without prior CVD, including diabetes and/or other traditional risk factors.6 While observational evidence suggests that individuals with advanced subclinical atherosclerosis40-42 and/or elevated lipoprotein(a)43-45 may derive net benefit (CVD risk reduction benefit outweighing risk of major bleeding) from primary prevention aspirin therapy, there are limited data specific for the primary prevention of stroke unlike for coronary heart disease (CHD). Class 3 recommendations (harm) for primary prevention aspirin therapy are provided for individuals with CKD and those ≥70 years of age without atherosclerotic CVD.Newly added to the 2024 Guideline is a 2b recommendation for the use of ticagrelor as a dual antiplatelet agent beyond 12 months, and up to 3 years, in addition to aspirin, for the prevention of ischemic stroke in those with stable CHD with low bleeding risk. This recommendation is derived from the PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis In Myocardial Infarction54) trial, where individuals with a history of myocardial infarction on background aspirin therapy randomized to receive 60 mg ticagrelor vs placebo experienced a 15% relative risk reduction of stroke (1.47% vs 1.94%).46 However, there was at least a 2.3-fold higher risk of major bleeding events (2.3% vs 1.1%) and a 2.8-fold higher risk of dyspnea (15.8% vs 6.4%) among individuals randomized to receive ticagrelor vs placebo.Additionally, recommendations regarding aspirin and/or direct oral anticoagulant therapy for individuals with decreased left ventricular (LV) systolic function (ejection fraction ≤35%-40%) and no atrial fibrillation (AF) or LV thrombus are provided. Based on evidence from the WARCEF (Warfarin vs Aspirin in Reduced Cardiac Ejection Fraction)47 and COMMANDER HF (A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure)48 trials, the 2024 Guideline does not recommend the use of anticoagulation among individuals with a reduced LV ejection fraction and no AF or LV thrombus due to an increased risk of major bleeding events (Class 3 recommendation, harm).Although not directly addressed in the 2024 Primary Prevention of Stroke Guideline, anticoagulation for primary prevention of thromboembolic stroke among individuals with AF is covered in the 2023 Diagnosis and Management of Atrial Fibrillation Guideline. Among individuals with AF who have an annual risk of thromboembolic stroke ≥2% (CHA2DS2-VASc ≥2 in men or ≥3 in women), selection of anticoagulation is recommended to be based upon the annualized stroke rather than the pattern of AF (paroxysmal, persistent, long-standing persistent, or permanent).49It is important for clinicians to have shared decision-making conversations with patients about their individualized stroke and bleeding risk, particularly for patients at very high stroke risk but whom have no other clear indication for antiplatelet or anticoagulant therapy.Colchicine
Start here