See also:
- American College of Rheumatology Clinical Practice Guidelines. From American College of Rheymatolgy. Accessed 3-19-2025
- JTF Practice Parameters and Guidelines from American Acacemy of Allergy, Asthma, & Immunology. Accessed 3-19-2025.
Today, I review, link to, and excerpt from Screening and Laboratory Diagnosis
of Autoimmune Diseases Using Antinuclear Antibody Immunofluorescence Assay and Specific Autoantibody Testing, Paul P. Doghramji, MD., 2016. Educational support provided by Quest Diagnostics, Inc.
All that follows is from the above resource.
Introduction
Autoimmune diseases occur when the immune system attacks the normal tissue within joints, vasculature, and other organ systems, causing inflammation, pain, diminished mobility, fatigue, and other non-specific symptoms.1 The strong overlap of signs and symptoms among the autoimmune diseases can lead to delays in diagnosis and appropriate treatment. According to a survey by the Autoimmune Diseases Association, it takes up to 4.6 years and nearly 5 doctor visits to receive a
proper autoimmune disease diagnosis.2Laboratory testing, in addition to clinical assessment, is necessary for differential diagnosis and disease classification
of autoimmune diseases. However, research shows that primary care physicians tend to overuse common autoantibody tests for autoimmune conditions, which can limit the positive predictive value and clinical utility of such testing.3
To facilitate appropriate referral to specialists,
if necessary, laboratory testing should be reserved for
patients who have signs and symptoms consistent with
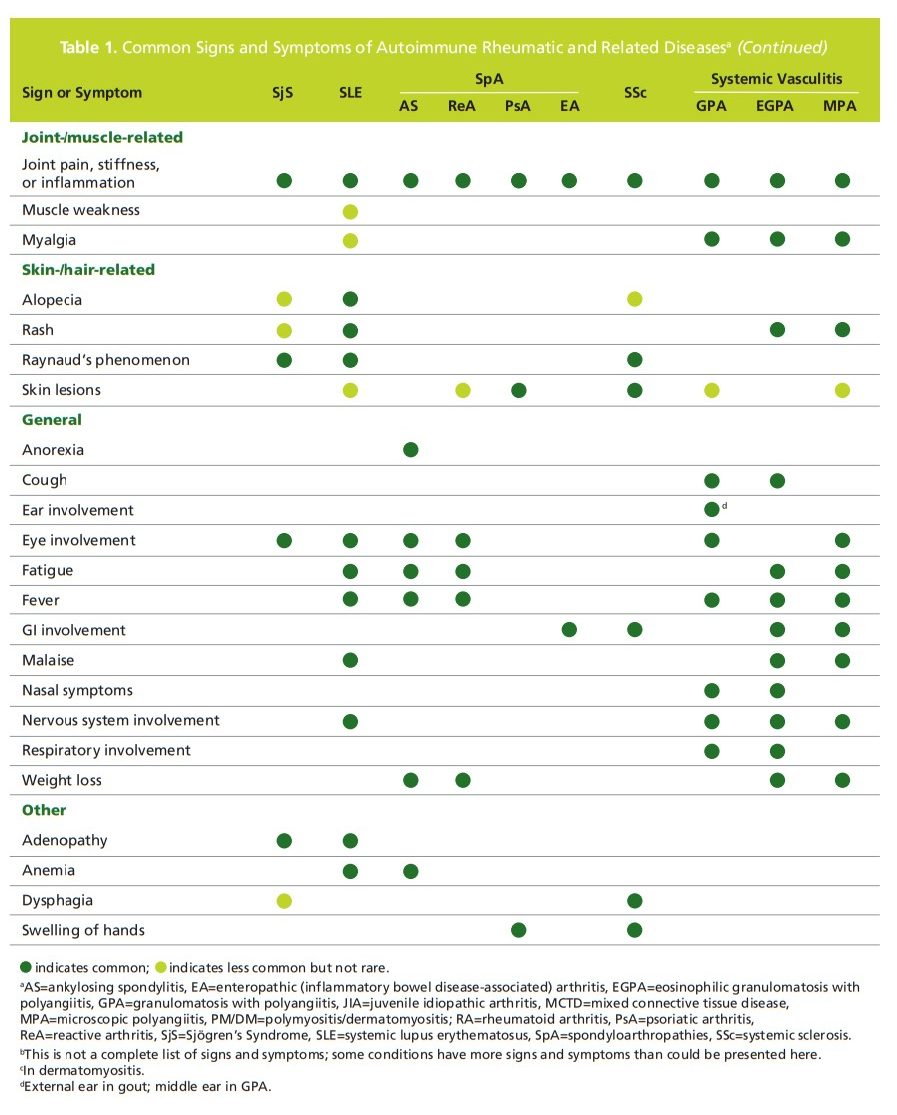
one or more autoimmune disease (Table 1).The antinuclear antibody (ANA) immunofluorescence assay (IFA) is a first-line screening test for patients with a suspected autoimmune disease. This test is the gold standard because of its high sensitivity compared to other assays.4,5 Positive results should prompt clinicians to continue investigating the cause of a positive ANA IFA and narrow the field of potential culprits. The following describes how ANA IFA in combination with specific
autoantibody testing can be used in the differential diagnosis of a suspected autoimmune disease.Titer And Pattern
If the ANA IFA screen is positive, testing for antibody titer and pattern can help evaluate the presence and type of autoantibody disease. ANA titers are determined by diluting the liquid portion of the blood sample in saline at a ratio of 1:40 to 1:1280. The titer is thus the highest dilution that yields a positive ANA result. Any titer above 1:40 is considered positive, and titers above 1:80 are consistent with an autoimmune disease. To assess the nuclear and cytoplasmic staining patterns of samples with positive results, patient antibodies react with indicator cells and the localization of patient antibodies is visualized by a second fluorescein antihuman IgG antibody evaluated under a fluorescence microscope. These patterns may provide additional information to rule out or further implicate a suspected condition and can guide the selection of additional testing for specific autoantibodies.
Laboratory screening and diagnostic testing for disease classification
The recommended ANA screen approach uses HEp-2 human tissue culture cells in an IFA. In this assay, the patient’s blood sample is mixed with HEp-2 cells fixed to a slide. ANAs present in the sample react with the cells and treatment with a fluorescent anti-human IgG antibody allows visualization of antibody binding under fluorescence microscopy. This test screens for a large number of known autoantibodies, approximately 150, directed against nuclear antigens and cell cytoplasm. A positive screen result is followed by evaluation of
antibody titer and pattern (consult side bar below).With a positive ANA IFA screen and titer, the clinician needs to determine the root cause of the positivity. This can be accomplished through a reflex to an algorithm of specific antibody tests to help identify autoantibodies associated with specific autoimmune diseases.
An ANA reflex algorithm tests for specific antibodies in a
clinically logical sequence. With a combination of ANA IFA
plus a reflex algorithm of specific antibody testing, positive
results automatically reflex to a tier of disease-specific
autoantibodies. Testing begins with the most prevalent
autoimmune diseases and continues until a positive result
is found, or all tests in the algorithm have returned a
negative result. This algorithm/reflex approach provides the
clinician with a rational approach to confirming a diagnosis
in a patient with a suspected autoimmune disease, with a
single blood draw.Figure 1 above illustrates an example of an algorithm approach with a set of reflexing tiers for suspected rheumatic disease, a more common group of autoimmune diseases.6-10 With this algorithm, samples with a positive IFA result are tested for five autoantibodies associated with systemic lupus erythematosus (SLE) and mixed connective tissue disease: dsDNA, chromatin (nucleosomal), Smith (Sm), ribonuclear protein (RNP), and Sm/RNP antiodies.
(In the cell, Sm and RMP proteins form a complex.) If the first tier yields a positive result, the results are reported and the testing stops. If the first tier of antibody testing is negative, the testing reflexes to the 2nd tier, which includes four autoantibodies associated with Sjögren’s Syndrome, systemic sclerosis, and polymyositis: SS-A, SS-B, Scl-70, and Jo-I antibodies. If a positive result is determined for any of these autoantibodies, the results are reported and the testing stops. If 2nd tier antibodies are negative, the testing continues with a reflex to 3rd tier. The 3rd tier includes two autoantibodies associated with CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia)and neuropsychiatric SLE: centromere B and ribosomal P antibodies.
Although a positive result in the 1st or 2nd tier will stop
the reflex testing to the next tier, it does not rule out the
presence of additional clinically relevant autoantibodies,
including those in the next tier or other autoantibodies
outside the algorithm. Additionally, negative results for all
three tiers do not rule out an autoimmune disease, as not
all autoimmune diseases are represented in this algorithm.
Note that the clinician can request full reporting through
all three tiers, especially if a rheumatic autoimmune disease
is strongly suspected.11Clinical suspicion and correlation are generally necessary
to proceed with additional testing for other specific
antibodies beyond the algorithm of the most common
rheumatic diseases. For example, a positive ANA IFA is a
diagnostic criterion for drug-induced lupus, polymyositis/
dermatomyositis, other forms of idiopathic inflammatory
myopathy, and oligoarticular juvenile chronic arthritis.
A positive ANA result is also consistent with organ-specific
autoimmune diseases, including thyroid diseases (eg,
Hashimoto’s thyroiditis, Grave’s disease), gastrointestinal
diseases (eg, autoimmune hepatitis, primary biliary
cholangitis, inflammatory bowel disease), and pulmonary
disease (eg, idiopathic pulmonary fibrosis).A positive ANA result alone is not diagnostic of an
autoimmune disease. The prevalence of ANAs in healthy
individuals is about 3-15%.11 The production of these
autoantibodies is strongly age-dependent, and increases to
10-37% in healthy persons over 65. Even healthy people
with viral infections can have a positive ANA, albeit for a
short time. Patients with infectious diseases may also test
positive for ANA. These include viral infections (hepatitis C,
parvovirus), bacterial infections (tuberculosis), and parasitic
infections (schistosomiasis). Certain medications and some
lymphomas may also cause a positive ANA.Case Studies
1 SLE
A 32-year-old Caucasian woman presented for an initial
visit because of soreness in her hands. This soreness began
six weeks previously, with no history of injury or prior pain
and soreness. The middle fingers of both hands (fingers
3 and 4) were swollen and tender, and she had trouble
making a fist and opening jars. Other joints were stiffer
than usual, especially her knees, ankles, and feet. She had
difficulty getting out of bed in the morning, with joint
stiffness and painful walking lasting many hours. The patient
denied fevers, chills, and any recent febrile illnesses, yet
reported feeling especially tired since this issue began. She
had been taking over-the-counter naproxen for “some”
relief. She denied rash, dry eyes/mouth, sun sensitivity, trouble swallowing, and cardiovascular symptoms.
The patient’s medical history was unremarkable. Her
medication list included birth control pills and she denied
any medication allergies. Her family history was significant
for thyroid disease (mother and older sister) and type 1
diabetes (father); social history included smoking ½ pack
per day for the past 18 years. She had no surgical history,
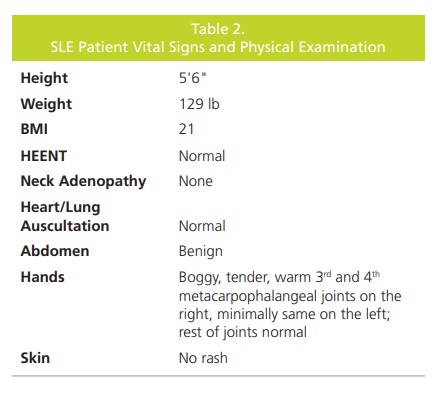
and a review of systems was unremarkable (Table 2).
The patient underwent laboratory testing using with the
ANA IFA with 3rd tier specific autoantibody reflex cascade
(Figure 1). The results were positive for ANA IFA at a titer of 1:160 with a mixed speckled and homogeneous pattern.
The 1st tier of testing was positive for dsDNA, chromatin,
and Sm antibodies, which pointed to a diagnosis of
SLE. The patient was then referred to a rheumatologist
who confirmed the diagnosis of SLE, and started her on
hydroxychloroquine. The primary care physician continues
to work collaboratively with the rheumatologist to manage
this patient.2 Sjogren’s Syndrome
A 58-year-old Japanese woman presented to the clinic
complaining of a very dry mouth and thirst that had been
going on for months and was now worsening. She was
worried about a diagnosis of diabetes mellitus (T2DM) due
to her family history (ie, parents had T2DM). The patient
had trouble speaking normally without constantly drinking
water and found it difficult to chew and swallow food.
Upon further questioning, she reported that she also had
dry eyes but no polyuria or weight loss, no swollen salivary
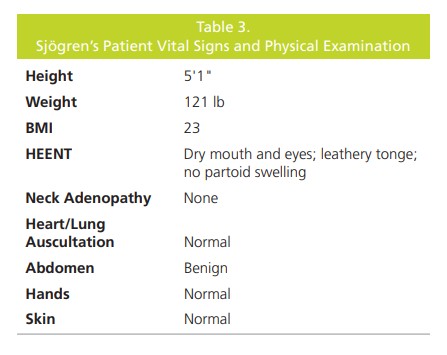
glands, no joint pain, and no skin changes (Table 3). Her
medical history was significant for the deep vein thrombosis
and for major depressive disorder, which had been treated
and was now in remission. She had no medications or
allergies to such. In addition to T2DM, her family history
was positive for hypertension (parents), hyperlipidemia
(parents), and rheumatoid arthritis (brother). The patient
had never smoked cigarettes and the review of systems
was unremarkable. On examination, the patient appeared
comfortable and in no pain; vital signs were normal.The results of routine laboratory testing (complete
blood cell count, sedimentation rate, and comprehensive
metabolic panel) were normal. The ANA IFA was positive
at a titer of 1:320 with speckled pattern. First tier cascade
testing (Figure 1) was negative, yet the 2nd tier was positive
for SS-A and SS-B. Rheumatoid factor was ordered and
was positive (≥ 14 IU/mL). The labs indicated a diagnosis of
Sjögren’s Syndrome.After the diagnosis of Sjögren’s Syndrome, the patient
was referred to a rheumatologist for further evaluation
and treatment. Consultation confirmed the diagnosis
and she was started on prednisone 10 mg daily and
hydroxychloroquine 200 mg daily. She was to follow-up
with the rheumatologist for further definitive treatment.
The primary care physician continues to work collaboratively
with the rheumatologist to manage this patient.3 Autoimmune Hepatitis
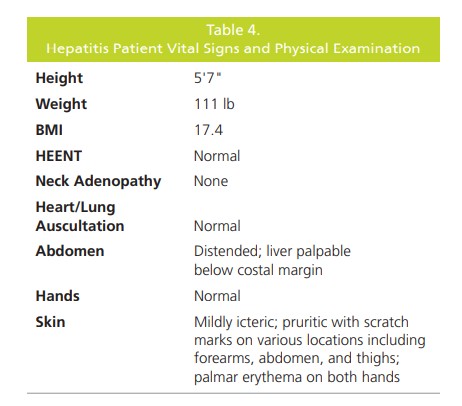
A 43-year-old woman presented with a 5-month
history of unintentional weight loss (13.2 lb), anorexia,
irritability, malaise and generalized pruritus. On physical
examination, she was mildly icteric with numerous scratch
marks, palmar erythema, and hepatosplenomegaly;
vital signs and physical exam were unremarkable (Table
4). Laboratory results showed a low hemoglobin level
(8 g/dL) with a normal white-cell count and an elevated
erythrocyte sedimentation rate (140 mm/h; normal ≤ 20
mm/h). The prothrombin time was prolonged, yet urea
and electrolytes, calcium and phosphate concentrations
were normal. Although the serum albumin was normal
(4.1 g/dL), the following were elevated: total serum
proteins (9.3 g/dL; normal range 6.1-8.1 g/dL), serum
bilirubin (1.8 mg/dL; normal range 0.2-1.2 mg/dL), alanine
transaminase (152 IU/L; normal range 7-55 IU/L), and
aspartate transaminase (164 IU/L; normal range 8-48 IU/L).
The alkaline phosphatase level was normal (83 IU/L; normal
range 45-115 IU/L). Her serum immunoglobulins showed
an increased IgG level (44 g/L; normal range 7.2-19.0 g/L)
with normal IgA and IgM levels.Antinuclear antibodies by IFA were strongly positive (titer
1:320) in a homogeneous pattern, and 1st tier autoantibody testing was revealed positive for only dsDNA antibodies.
The ANA IFA also showed cytoplasmic staining of actin
elements. Testing was positive for antibodies to smooth
muscle consistent with autoimmune hepatitis. Hepatitis B
surface antigen and hepatitis C antibody was absent and
alpha fetoprotein was not detected. The immunological
picture and absence of hepatitis B and C infection strongly
favored a diagnosis of autoimmune hepatitis. The ANA titer 1:320 was a definitive diagnosis. The patient was
therefore started on prednisolone (30 mg/day) and vitamin
K, and showed dramatic improvement. Her serum bilirubin,
transaminases, and prothrombin time returned to normal
over the next two weeks. A diagnostic liver biopsy showed
chronic active hepatitis with cirrhosis. She was continued
on prednisolone (15 mg/day) and is fully reassessed every
six months, including repeat liver biopsy as appropriate.Conclusion
An ANA IFA cascade with reflex to specific testing
has clinical significance in the proper setting. A positive
ANA IFA by itself can show pre-clinical autoimmune
disease, yet utilizing the ANA IFA cascade with reflex
to specific testing can lead to early diagnosis and early
treatment of potentially devastating diseases, putting some
patients in remission. Test results should be interpreted in
a clinical context that includes a history and physical, basic
chemistry panel, imaging studies, and assessment of signs
and symptoms.