Today, I review, link to, and excerpt from The American Society Of Echocardiography‘s Recommendations for the Assessment of Carotid Arterial Plaque by Ultrasound for the Characterization of Atherosclerosis and Evaluation of Cardiovascular Risk: From the American Society of Echocardiography. [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. J Am Soc Echocardiogr. 2020 Aug;33(8):917-933. doi: 10.1016/j.echo.2020.04.021. Epub 2020 Jun 27.
Comment in
-
J Cardiothorac Vasc Anesth. 2021 Apr;35(4):987-990. doi: 10.1053/j.jvca.2020.12.006. Epub 2020 Dec 16.PMID: 33431270 No abstract available.
-
Reliability, Reproducibility, and Advantages of Measuring Carotid Total Plaque Area.
J Am Soc Echocardiogr. 2022 May;35(5):530-532. doi: 10.1016/j.echo.2021.12.016. Epub 2022 Jan 25.PMID: 35085719 No abstract available. -
J Am Soc Echocardiogr. 2022 May;35(5):532. doi: 10.1016/j.echo.2022.01.015. Epub 2022 Feb 5.PMID: 35134520 No abstract available.
There are 97 similar articles in PubMed.
The above article has been cited by 123 articles in PubMed.
All that follows is from the above resource.
Abstract
Atherosclerotic plaque detection by carotid ultrasound provides cardiovascular disease risk stratification. The advantages and disadvantages of two-dimensional (2D) and three-dimensional (3D) ultrasound methods for carotid arterial plaque quantification are reviewed. Advanced and emerging methods of carotid arterial plaque activity and composition analysis by ultrasound are considered. Recommendations for the standardization of focused 2D and 3D carotid arterial plaque ultrasound image acquisition and measurement for the purpose of cardiovascular disease stratification are formulated. Potential clinical application towards cardiovascular risk stratification of recommended focused carotid arterial plaque quantification approaches are summarized.
Keywords: Atherosclerosis; Carotid plaque; Risk stratification.
Copyright © 2020 American Society of Echocardiography. Published by Elsevier Inc. All rights reserved.
Abbreviations and Acronyms
ASE (American Society of Echocardiography)
CIMT (carotid intima-media thickness)
IPN (intra-plaque neovascularization)
MACE (major adverse cardiovascular events)
MRI (magnetic resonance imaging)
PDA (pixel distribution analysis)
PET (positron emission tomography)
UEA (ultrasound enhancing agent)
Background
Atherosclerotic cardiovascular disease (CVD) remains the leading global cause of morbidity and mortality.1 Ultrasound imaging of the carotid artery has the ability to provide a unique “window” into the identification of a patient’s underlying cardiovascular risk.2 The presence and degree of atherosclerosis, as defined by plaque presence detected in the carotid arterial system, has been used to estimate and classify or reclassify an individual’s cardiovascular risk. Beyond overall risk stratification, carotid atherosclerosis is also a known predictor of other CVD events, such as stroke resulting from luminal vessel stenosis and plaque rupture.3,4It is now recognized that CIMT may represent more than one distinct morphologic process while plaque primarily reflects atherosclerosis. CIMT may predominantly reflect the presence of cardiovascular risk factors (such as hypertension), whereas carotid plaque, a sub-intimal process, may be more reflective of atherosclerosis, as it correlates with overall atherosclerotic burden in the coronary vascular bed.8,9 The high prevalence of carotid atherosclerosis in subjects with an otherwise low Framingham risk score has potential implications for screening of subclinical atherosclerosis.8 Thus, quantification of carotid arterial plaque has emerged as an important tool for CVD risk stratification beyond what is offered by CIMT. This document focuses on the methods to quantify carotid arterial plaque, when present, for the purpose of risk stratification.CIMT can still provide useful information even if no plaque is present. Currently, CIMT assessment is well described in “The ASE Consensus Statement on the Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Risk”.5 The CIMT related recommendations from this consensus continue to be endorsed by this writing panel. Henceforth, recommendations under the following headings from the previous consensus will not be revisited in the current document: 1) Rationale for Carotid Ultrasound to Identify Subclinical Vascular Disease, 2) Instrumentation, Display and Scanning Technique, 3) Reporting of Carotid Ultrasound Study Results, and 4) Training and Certification of Sonographers and Readers.5 The current document complements the previous consensus in its provision of a standardized approach to defining and quantifying carotid arterial plaque by ultrasound beyond the technical approach to CIMT measurement. Specifically the previous consensus did not provide a standardized approach to plaque quantification. Since the publication of the previous consensus in 2008, carotid ultrasound technology has advanced tremendously, first from the widespread availability of a dedicated three-dimensional vascular ultrasound probe, and now more recently, following the release of a 3D matrix array probe for carotid ultrasound with concomitant analysis software. The current document is the first to provide systematic recommendations for standardization of the quantification of carotid arterial plaque for the purposes of CVD risk stratification.Scope
This consensus statement provides recommendations for the 2- and 3-dimensional quantification of carotid arterial plaque by ultrasound for the basis of CVD risk stratification. Emerging techniques, including the role of ultrasound enhancing agents (UEA) for assessment of intraplaque neovascularization and composition analysis, are also discussed.Definition of Plaque – Protuberant and Diffuse Types
Carotid arterial atherosclerosis is thought to develop beneath the intimal layer in the sub-intima. In contrast, the medial layer is subject to non-atherosclerotic medial hypertrophy commonly induced by aging and hypertension. Since the largest portion of CIMT (∼99% in healthy individuals and ∼80% when diseased) consists of the medial layer, CIMT has not been shown to consistently add to CVD risk prediction. Carotid plaque, on the other hand, represents the atherosclerotic process itself, and starts in the intimal layer and has thus been shown to predict CVD events better than CIMT.7,10 Despite this difference in carotid arterial phenotypes, which have been used to describe associations with CVD events and risks4,11, it can be difficult to discern medial thickening from diffuse atherosclerotic plaque. Though some atherosclerotic plaques are discrete lesions that can be easily distinguished from the surrounding wall, plaque can also be eccentric and spread over the surface of the wall, appearing indistinct from the media. In such cases it is difficult to determine whether there is simply medial thickening present or eccentric, diffuse plaque. Thus, arbitrary definitions to define the presence of diffuse plaque beyond a certain CIMT threshold have been proposed.12 Adding to this complexity is the debate as to whether the transition from increased CIMT to plaque formation is a continuous process13, or if CIMT and plaque are truly separate phenotypes.14A commonly reported threshold value to define diffuse plaque is a CIMT value greater than 1.5 mm or a focal intimal medial thickening of greater than 50% of the surrounding area.15,16 However, confusion occurs because ultrasound resolution now allows for the visualization of distinct protuberant plaque lesions that could be smaller than this threshold value. Furthermore, even the threshold CIMT value signifying plaque varies among studies. For example, in one study, plaque was defined as a focal thickening of the intima-media greater than 1 mm, protruding into the lumen, that was at least twice as thick as the surrounding normal CIMT, thus providing varying definitions of plaque ranging from 0.5 mm to >1.5 mm.17 In comparison, another study defined plaque as CIMT >1.2 mm.18 In contrast, the European Mannheim consensus defined plaque as a focal thickening that encroaches into the lumen by 0.5 mm or by 50% of the surrounding intimal-medial thickness or where CIMT is >1.5 mm.19Our writing panel selected a CIMT threshold value signifying plaque that is slightly more conservative than the Mannheim consensus16,19 by recommending ≥1.5 mm (vs >1.5 mm) as the cut-off CIMT threshold value for the presence of diffuse plaque. This newly established Plaque Grading Consensus, described in detail below, now allows for the identification and characterization of protuberant plaque lesions smaller than the CIMT threshold value for identifying diffuse plaque. In other words, we recognize that plaque lesions smaller than 1.5 mm can be highly resolved with today’s technology. Advances in ultrasound now allow for identification of such small lesions in exquisite detail, allowing for both quantification and even potential analysis of composition. Thus this modern grading system sets a framework for continued outcomes-based research across the spectrum of plaque lesion shapes, sizes, and types.Recommendation #1: We recommend that carotid arterial plaque visualized by ultrasound (with or without use of an ultrasound enhancing agent [UEA]) be defined in one of the following 2 ways: 1) any focal thickening thought to be atherosclerotic in origin and encroaching into the lumen of any segment of the carotid artery (protuberant-type plaque) or 2) in the case of diffuse vessel wall atherosclerosis, when carotid intima-media thickness (CIMT) measures ≥1.5 mm in any segment of the carotid artery (diffuse-type plaque).Recommendation #2: We recommend the evaluation of both protuberant and diffuse types of carotid arterial plaque for cardiovascular risk stratification and the serial assessment of atherosclerosis.Recommendation #3: We recommend that first, the carotid arterial wall be visually scanned for the presence of protuberant plaque, and if absent, then carotid intima-media thickness (CIMT) measurement be performed to identify the presence of diffuse plaque (defined as CIMT ≥1.5 mm). If performed, CIMT should be measured as described in the ASE Consensus Statement on the Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Risk.5Clinically Significant Carotid Arterial Plaque or CIMT
It is recognized that in some centers, repeat evaluation of CIMT in the absence of plaque is considered if CIMT is >75th percentile for age, race, and gender.20 Despite the lack of evidence surrounding the frequency of repeat testing, an interval for repeat testing of 2-5 years has been utilized in population studies, although published evidence suggests that more frequent CIMT measurements could increase the precision of the assessment of CIMT progression.21 Methodological limitations of this study notwithstanding, our expert panel recommends against serial CIMT measurements for CVD risk stratification especially when not meeting the threshold for diffuse plaque (≥1.5 mm). We recognized that based on limited or anecdotal evidence, there may be value in serial CIMT measurements in the hands of some experts for research,22,23, monitoring of progression or regression in specific cases 24,25, and as a potential tool to alter patient behavior.26 Additionally, it is possible that over time, an individual patient may have a CIMT value that has increased to ≥1.5 mm, signifying the development of diffuse atherosclerotic plaque by our definition; however the clinical utility of such long term CIMT monitoring is not yet established.We have suggested that a CIMT ≥1.5 mm be considered a clinically significant lesion for patients less than 65 years of age. The thickness (also known as height in the long axis view) of a carotid arterial plaque lesion, whether it is protuberant or diffuse, was chosen as the initial measure to define plaque because of its widespread availability and because this variable can be measured in both protuberant or diffuse types of plaque lesions (Figure 1). Additional quantification techniques such as area and volume apply mostly to protuberant-type plaque lesions, and are difficult to define in atherosclerotic lesions that are diffusely layered along the intimal wall. Such lesions may be focal or diffuse wall calcification layered in a concentric or eccentric manner and may represent atherosclerotic or non-atherosclerotic processes. Accordingly, our panel suggests the grading system for both protuberant and diffuse plaque lesions as described in Figure 1.Figure 1 Plaque Grading Consensus: carotid medial thickening and intimal plaque. Carotid intimal-medial thickening is thought to involve thickening of the medial layer whereas plaque is thought to be an intimal process as suggested in this schematic. However, diffuse intimal thickening may also occur that is difficult to distinguish from a medial process, and though not protuberant, eccentric or concentric thickening of 1.5 mm or greater is suggested to be a plaque equivalent by this system.The grading system does not reflect the degree of vesselocclusion but attempts to standardize the size of an individual plaque lesion for the purpose of comparison across studies. It is important to note that Grade I characterization applies only to small protuberant types of plaque lesions. This is because, though ultrasound can now resolve such small protuberant lesions, if the plaque is non-protuberant (diffuse or eccentric) and less than 1.5 mm in thickness, it is currently not possible to distinguish whether this is entirely due to medial thickening or is intimal. However at a CIMT value of 1.5 mm or greater (Grades II and III), this framework attributes the thickness to be due to diffuse atherosclerotic plaque (mostly intimal rather than medial) and is thus considered a plaque equivalent. The Grades II and III measurements are applied to obviously protuberant plaque in the same manner for simplicity (Figure 1).Recommendation #4: We recommend against serial carotid intima-media thickness (CIMT) measurements in an asymptomatic patient. Repeat measurements are not recommended unless the Grade and (CIMT) meets criteria for diffuse-type plaque (Grades II or III, and CIMT ≥1.5 mm) in which case it is a plaque equivalent.Quantification Methods
Two-Dimensional Techniques for Quantifying Plaque
Interest in plaque quantification significantly grew when it was discovered that the presence or absence of plaque conferred additional advantage to risk stratification beyond that provided by CIMT alone (ARIC Study).27 It was recognized that if simply the presence or absence of plaque re-stratified patients beyond traditional risk factors, then examining the amount of plaque may further personalize a patient’s risk assessment.An early, highly variable approach, was to quantifying plaque using a visual plaque score, which evaluated and reported the total number of plaques or affected segments occurring anywhere in the common carotid artery (CCA), carotid bulb, or internal carotid artery (ICA) in any wall (near, far, lateral). More precise methods include 2-dimensional (2D) quantification techniques such as the maximal plaque height or thickness. Another common 2D approach has been the calculation of the plaque area where the area of one or multiple plaques is measured, and the total value reported. The advantages, disadvantages, and data with respect to outcomes for these 2D quantitative measures are briefly summarized followed by recommendations for performance.Plaque score
The plaque score is a semi-quantitative approach where the total number of sites containing plaque along the CCA, carotid bulb, and ICA are visualized and summed. This approach varies greatly among studies – some investigators count plaque lesions in any visualized segment, whereas others count only the lesions seen in easily identified segments such as the distal first centimeter (cm) of the CCA, bulb, and proximal ICA. Two important analyses from the Rotterdam Study, a prospective, population-based cohort that aimed to determine the occurrence of CVD (among other disease) in elderly people, calculated plaque score using a unique process. Both studies (n1=4217 and n2=6389) measured the presence of carotid plaques at 6 locations in the carotid arteries (two sides each of the CCA, bifurcation, and ICA). The total plaque score ranged from 0 to 6 and was calculated by adding the number of sites at which a plaque was detected, divided by the total number of sites for which an ultrasound image was available, and multiplying by 6 (maximum number of sites).28,29 In the second study (n=6389), patients with a plaque score of 0, 1, 2, and ≥3 points were considered to have no, mild, moderate, or severe carotid atherosclerosis, respectively.28,29For the purposes of risk prediction, and in order to attempt standardization, we recommend that if a plaque score is being calculated, then lesions limited to the distal 1 cm of the CCA, bulb, and proximal 1 cm of the ICA be included in the counting.29 The need for further effort toward standardization of the plaque score is recognized.Clinical studies have shown an association between plaque score and incident CVD. In the Three-city study of 5895 individuals (aged 65-85 years) free of CVD, the presence of plaque in one site was associated with a hazard ratio (HR) of 1.5 [95% confidence interval (CI) = 1.0-2.2] while the HR for plaque at ≥ 2 sites was 2.2 (95% CI = 1.6-3.1; pfor trend < 0.001).30 The addition of plaque information to traditional risk factors also improved the area under the curve (AUC) for CVD prediction from 0.728 to 0.745 (p = 0.04) with a net reclassification index (NRI) of 13.7%. Another study showed that in 367 men (mean age 78±4 years) the hazard ratio for mortality over 4 years increased from 2.89 for a plaque score of 1-2 to 4.53 for a plaque score of 7-12.31Disadvantages
The plaque score is a semi-quantitative method that simply counts the number of lesions. This method does not consider additional parameters such as the size of an individual plaque lesion which would then otherwise better reflect the overall extent of atherosclerosis. Additionally, because the number of plaque lesions at distinct sites is counted in this method, the score may not be clear as to whether two distinct plaque lesions are in fact contiguous, thus providing an overestimation. Similarly, there may be a significant degree of protuberant contiguous plaque, but if this is just one large lesion, the plaque score will underestimate the extent of atherosclerosis.Plaque height/thickness
Carotid plaque thickness or height may be considered a variation of the maximal CIMT measurement but differs in that it represents the degree to which the plaque protrudes in a radial manner from its origin, along the vessel wall, into the lumen. To conduct this measurement, some investigators suggest electrocardiographic gating in order to measure at the same phase of the cardiac cycle. Typically, cross-sectional (transverse) sweeps to evaluate for the presence of plaque are made and then once a plaque lesion is identified, electronic calipers (available with most software) are placed beginning along the origin of the plaque at the vessel wall, into the lumen (at right angles to the wall) along the most protuberant aspect of that particular plaque. The maximum plaque height or thickness among all identified plaque lesions, visualized in both the right and the left carotid arteries, is then reported. It is important to note that studies utilizing this method do not sum the plaque heights measured but report the single largest plaque height measured from any plaque identified in the patient. Plaque height measured in this manner is highly reproducible. The intra-class correlation coefficient for inter-observer and intra-observer reliability were 0.77 and 0.94, respectively, in the Northern Manhattan Study (NOMAS).32 Variability in this method is related to identification of the location within the vessel wall, where plaque height measurement should begin. To reduce variability across studies, we recommend beginning the measurement at the adventitial-medial layer, similar to CIMT measurement.Association of Plaque Height with Outcomes
Maximum plaque height ≥1.54 mm has been shown to be associated with significant coronary artery disease.33 Similarly, asymptomatic subjects with advanced atherosclerosis of the carotid artery (carotid plaques with a CIMT ≥ 3.5 mm and flat carotid plaques with a CIMT > 2 mm) have been shown to have a high risk of coronary heart disease.34 In the High Risk Plaque (HRP) Bioimage study, hazard ratios for major adverse cardiovascular events (MACE) for the maximum carotid plaque thickness were 1.96 (95% CI 0.91-4.25, p = 0.015) for primary MACE [cardiovascular death, myocardial infarction, or stroke] and 3.13 (95% CI 1.80-5.51, p < 0.001) for secondary MACE (all-cause death, MI, ischemic stroke, unstable angina, or coronary revascularization).35Advantages
The main advantage of using maximum plaque thickness is that it is a precise quantitative measurement but remains very simple to perform using a standard 2D linear probe. It is usually easy to visualize the largest plaque from any segment of the right and left common carotid arteries, and then one only needs to measure the height of the largest plaque. Furthermore, this method has supportive data demonstrating association with outcomes.36Disadvantages
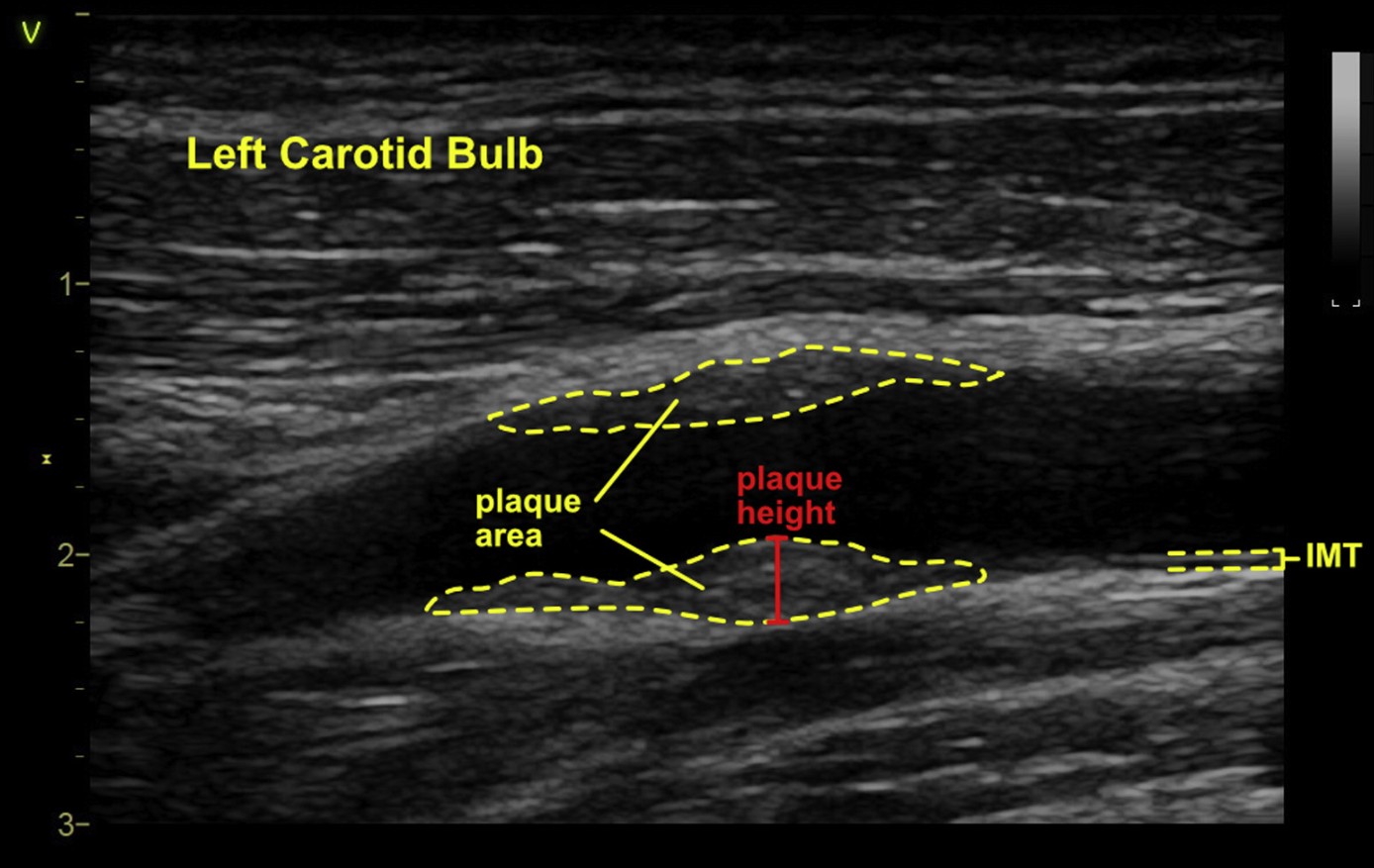
Some of the same disadvantages associated with CIMT measurement occur with measurement of plaque thickness as well. It is recognized that common to all two-dimensional ultrasound techniques, the maximal plaque height may be out of plane and underestimated, or overestimated if scanning is not performed through the center of the artery.37 Whether a long- or short-axis view of the artery is obtained, the maximal plaque height will be an underestimate if the acquisition is not through the plane bisecting the largest or most protuberant component of the plaque. Another disadvantage of 2D plaque height is that thickness may not truly reflect the burden of disease, because such lesions also have width. For example, a very small or minor plaque lesion that is protuberant, will have a higher thickness value compared with an extensive and heavily layered plaque that may not be as protuberant but is more eccentric and has a greater overall volume burden. Many of these disadvantages can be overcome through the acquisition of a three-dimensional image if technology is available (described in detail below). On balance, the writing group concluded that there are sufficient advantages (especially simplicity) to performing 2D plaque thickness measurement whether this is acquired from a 2D or 3D imaging probe.Recommendation #5: We recommend that plaque thickness (also known as height) be measured as the initial 2-dimensional approach∗ for quantification of carotid ultrasound plaque.∗Though plaque height is often measured from 2D images, it can be obtained from a 3D image acquisition when available, to overcome the out-of-plane limitations of 2D imaging.Recommendation #6: The maximal plaque height should be measured from the side in which a plaque is detected (unilateral) or from both the right and left carotid arterial segments (bilateral) using a caliper placed at the adventitial plane∗∗, and extending into the center of the lumen at right angles to the vessel wall. For the purposes of standardization, this measurement should be taken from any segment of the long and short axis of the carotid artery (bulb, ICA, CCA) and the view and segment reported accordingly.∗∗This measurement begins at the same plane as where the carotid intima-media thickness (CIMT) measurement begins in order to be consistent with defining plaque beyond the CIMT threshold of >1.5 mm.Plaque Area
Carotid plaque area is the most advanced of the 2D quantification methods. This method begins with a manual sweep of the carotid artery, typically scanning the artery in cross section, to initially identify plaque lesions. Once identified, the lesion is imaged in a manner that would provide its largest area, usually in the longitudinal view (long axis) of the carotid artery. The lesion is then manually traced using basic software present in most analysis packages. A more advanced analysis can occur if semi-automated or automated software is available. For example, in the High Risk Plaque study protocol, the media-adventitia boundary and the lumen-intima boundary are marked manually and then the plaque area is traced in an automated manner. If multiple plaques (same artery or bilateral) were present, they would be summed to define the total plaque area. The semi-automated method has been validated with CVD outcomes.38Advantages
Carotid plaque area provides the most information on plaque “quantity” or burden that is available through 2D ultrasound techniques. For example, compared with plaque height, plaque area would be a better indicator of overall plaque burden in distinguishing an eccentric or large layered plaque from a small, protuberant plaque. There are good data demonstrating the association with incident events and also response to therapy.39,40 Measurement can be performed with commercially available software.Disadvantages
Two-dimensional imaging measurements are affected by the imaging plane and can introduce variability into area measurements. Cross-sectional imaging helps mitigate this to an extent. Additionally, judging the extent of plaque, i.e., the area surrounding the maximal plaque thickness can be subjective and hence associated with inherent measurement errors. Other general but important limitations associated with standard 2D imaging of arterial plaque include the operator dependence of this method, variable image quality, out-of-plane registration errors and the time requirement, limiting its use in clinical practice.41 On balance, the writing group concluded that the disadvantages of measuring plaque area outweigh the current limitations of 2D height measurement, and we have not identified this approach as a recommended technique (See recommendations #5 and #6). Figure 2 provides a summary of current 2D methods, including plaque height and area.Figure 2 2D methods of plaque assessment. Two-dimensional methods of quantifying arterial plaque, including plaque area and plaque height. Intimal-medial thickening is also shown for demonstration but is suggested to be measured in the absence of plaque. As demonstrated in this figure, plaque thickness is measured beginning from the adventitial plane (same plane as where CIMT begins). It is recognized that in some cases the plaque may be mostly intimal, appear distinct from the underlying medial layer, and not extend fully to the medial-adventitial border, however the measurement should still begin from this medial-adventitial plane for the purposes of standardization.Application of Carotid Arterial Plaque Imaging in Clinical Practice
Primary prevention/asymptomatic patients
It appears reasonable to combine established risk scores with plaque imaging. The 2016 European Society of Cardiology Guidelines on Cardiovascular Disease Prevention have included plaque detection as a modifier in cardiovascular risk assessment (class IIb, level B) after the initial assessment has been performed using established risk scores.91 The same recommendation (class IIb, level B) is given for coronary calcium scoring. A risk modifier is likely to have reclassification potential.91 Previous work has showed that ultrasound examination allowed improved identification of individuals who could be targeted for prophylactic medical intervention.92 However, the 2019 American Heart Association/American College of Cardiology guidelines93 and Canadian Guidelines for the Management of Dyslipidemia for the Prevention of in the Adult94 have only included coronary calcium scoring but not carotid plaque imaging as a risk modifier. Further studies are needed to obtain more evidence for using plaque assessment as a risk modifier and to define which groups benefit from a combined assessment. This document facilitates this work by recommending standard plaque assessment approaches for comparison across studies. In the assessment of asymptomatic at-risk patients, we suggest a stepwise approach to cardiovascular risk stratification using plaque grading via a focused carotid vascular ultrasound and subsequent 2D or 3D plaque quantification (Figure 7).Figure 7 Cardiovascular risk stratification pathway using plaque grading by 2D/3D ultrasound. ∗Risk Score (Asymptomatic At-Risk Population) adapted from Greenland et al. (2010)95: Low Risk: <6% Framingham Risk Score (FRS), Intermediate Risk: Grey Area (6-10% to ≤20% FRS), High Risk: ≥20% FRS. ∗∗European Guidelines on CVD Prevention in Clinical Practice (Class IIb, Level B); adapted from Piepoli et al. (2016).91 ∗∗∗3D quantification is recommended to determine the maximal plaque height value from either the left or right carotid arterial bulb. 3D volume should also be measured however, a threshold volume has not yet been determined in large outcome studies. The value of 0.08 ml was found to be associated with significant CAD in a selected population. This algorithm requires updating following further 3D plaque volume investigations.Symptoms suspicious of coronary artery disease, but normal non-invasive tests
The functional tests for assessment of coronary artery disease (e.g., stress electrocardiogram, stress echocardiography, stress MRI, and nuclear imaging) cannot detect coronary artery obstruction between 50 and 70%. Although the short-term prognosis of patients with a normal stress test is good, direct assessment of atherosclerosis may be helpful for long-term prognosis and prevention. Recent studies suggest that carotid plaque imaging in patients with normal stress tests provides improved prognostic information: patients without plaque have an excellent prognosis, whereas patients with a normal imaging test for myocardial ischemia, but atherosclerotic plaques in the carotid artery, may benefit from more aggressive medical treatment.96-100 The combination of carotid plaque assessment with stress testing is a promising area offering enhanced risk stratification. Further multi-center confirmation will allow for consideration of practice recommendations in the future. Monitoring and adjusting treatment of atherosclerosis by repetitive plaque measurements appears to be an attractive application that needs further development of reliable, user friendly tools to assess the carotid plaque burden and clinical studies to define the time intervals and changes in dosing of statins and other medications. The writing panel calls for further development and study of application tools for the integration of carotid plaque assessment into existing risk stratification algorithms and testing.Summary
This document provides recommendations for the definition and quantification of carotid arterial plaque. A framework for grading atherosclerotic plaque based on thickness is provided to facilitate comparison across studies and monitoring of patient outcomes. Of 2D techniques, plaque height is recommended as the preferred approach and formulated to promote standardization. Three-dimensional volumetric ultrasound quantification is preferred when available, and a recommendation for plaque volume quantification is provided to promote standardization. The role of composition analysis to assess plaque vulnerability and tissue types continues to emerge. The important role of plaque analysis by ultrasound in cardiovascular risk stratification continues to require innovative approaches to dissemination of this knowledge and greater efforts toward translation to practice.NOTICE AND DISCLAIMER: This report is made available by ASE as a courtesy reference source for members. This report contains recommendations only and should not be used as the sole basis to make medical practice decisions or for disciplinary action against any employee. The statements and recommendations contained in this report are primarily based on the opinions of experts, rather than on scientifically-verified data. ASE makes no express or implied warranties regarding the completeness or accuracy of the information in this report, including the warranty of merchantability or fitness for a particular purpose. In no event shall ASE be liable to you, your patients, or any other third parties for any decision made or action taken by you or such other parties in reliance on this information. Nor does your use of this information constitute the offering of medical advice by ASE or create any physician-patient relationship between ASE and your patients or anyone else.