Today, I review, link to, and excerpt from Trends in clinical studies evaluating neurofilament light chain as a biomarker [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Biomark Med. 2025 Sep;19(17):813-823. doi: 10.1080/17520363.2025.2562546. Epub 2025 Sep 16.
There are 101 similar articles in PubMed.
All that follows is from the above article.
- Abstract
- 1. Introduction
- 2. Clinical Applications
- 3. Analytical Challenges
- 4. Metrological Challenges
- 5. Clinical Challenges
- 6. Conclusions
- Acknowledgments
- Author Contributions
- Institutional Review Board Statement
- Informed Consent Statement
- Data Availability Statement
- Conflicts of Interest
- Funding Statement
- Footnotes
- References
- Associated Data
1. Introduction
Here, we will summarize the current context of Nf-L, highlighting the different associated diseases, biofluids and diagnostic purposes as well as the current quantification techniques showing great potential for clinical implementation. Then, we will focus on the remaining challenges around this biomarker. We will first describe the measurement variability and the pre-analytical component affecting Nf-L levels in biofluids. Furthermore, we will focus on the metrological challenges such as developing RMP and CRM and on characterizing the measurand, and how mass spectrometry could help to address them. These considerations are critical to achieving standardization of the assays and the development of assay calibrants in order to ensure long-term success. Finally, we will also describe the remaining clinical challenges, including the different pathophysiological factors affecting Nf-L measurements and the need for defining reliable clinical thresholds prior to implementation in clinical routines.
2. Clinical Applications
Nf-L is a well-established biomarker in the clinic for assessing axonal damage. However, it is not specific to a disease as its level increases in several pathologies such as multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), dementias including Alzheimer’s disease (AD), fronto-temporal dementia (FTD) and other diseases [4,5,6,7,8,9]. Nf-L levels are also higher in days following a stroke or after a TBI [4,5]. Therefore, if this biomarker is to be used for diagnostic purposes, it should be complemented with disease-specific biomarkers.
Nevertheless, in some instances, Nf-L can differentiate one disease from another and help make differential diagnoses. This is the case with Parkinson’s disease (PD) and FTD.
Additionally, its utility has been highlighted not only for diagnostic but also for prognostic purposes and for evaluation of treatment efficiency. Indeed, several studies have shown that an increase in Nf-L levels correlates with disease evolution. For instance, in patients suffering from MS, a rise in Nf-L concentration can predict relapses. It is also the case for AD patients. Indeed, different values in Nf-L measurements were observed between cognitively impaired and AD dementia patients [7,9]. In addition, increased Nf-L levels were associated with cognitive decline in the AD continuum and can predict disease progression. It also correlates highly with imaging and cognitive assessment results [14,15].
Moreover, as with many other NDD biomarkers, Nf-L levels increase before any clinical manifestations. A rise of Nf-L can be seen as early as 10 years in sporadic AD before any clinical symptoms appear [16]. In familial AD, it could even be 22 years before as demonstrated by Quiroz et al. [17] in a study comparing presenilin 1 E280A mutation carriers with non-carriers. In addition, the yearly increase in Nf-L was much more observable in mutation carriers. The average annual change was 15.26 pg/mL for mutation carriers vs. 1.99 pg/mL for non-carriers.
Finally, monitoring Nf-L in clinical trials showed an enormous potential to determine treatment efficiency. Raket et al. [18] supported the use of Nf-L in clinical trials for monitoring the cognitive decline occurring in the short term. Furthermore, in several studies, the effect of treatments for multiple sclerosis (MS) was evaluated by monitoring Nf-L levels in the blood. Kuhle et al. [19,20] described the effect of fingolimod and alemtuzumab in MS patients using serum Nf-L measurement for monitoring the efficacy of treatment. In the first study, they showed that Nf-L was sensitive to the treatment and indicated a decrease of 36% after 6 months in patients treated with fingolimod in two trials compared to a placebo [19]. In the second study, they monitored Nf-L levels during treatment with alemtuzumab and 7 years post-treatment. They showed that Nf-L levels decreased at the beginning of the treatment and were stable over the 7 years of follow-up [20].
3. Analytical Challenges
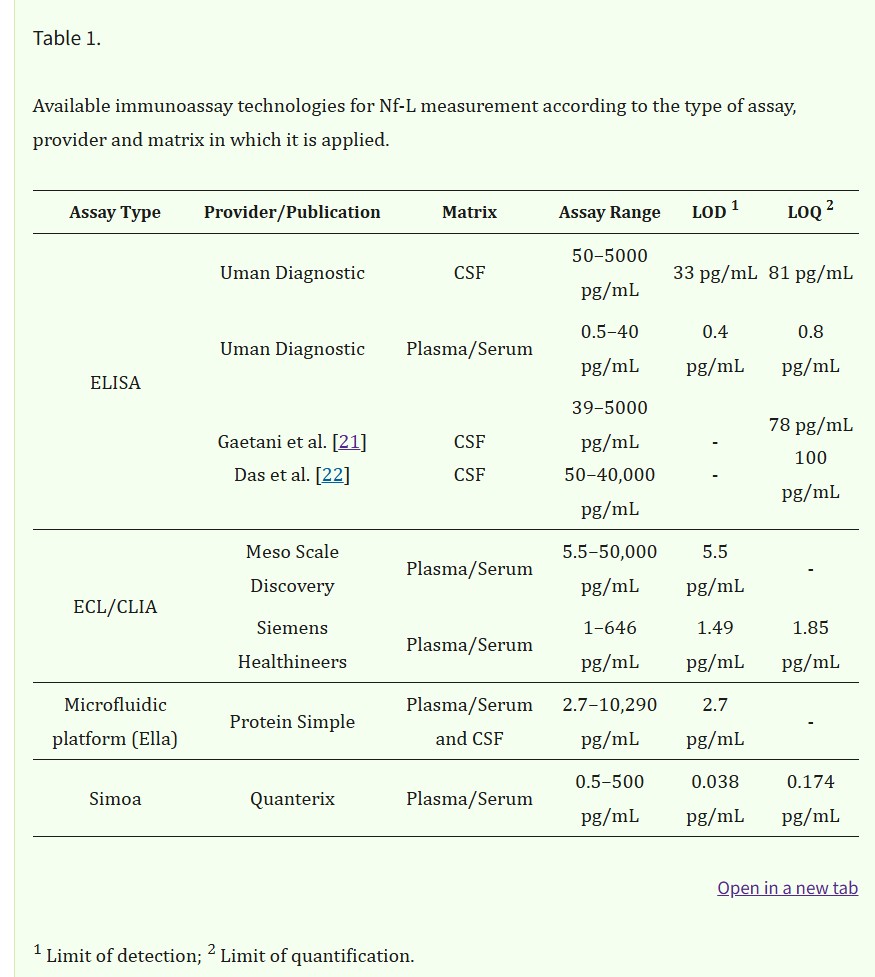
Nf-L quantitation in body fluids is performed currently by immunoassays using various technologies (Table 1) but some challenges in measurement remain.
Pre-analytical variations including the type of matrix or the sampling procedure and sample storage are important considerations, which will be discussed below in greater detail.
3.1. Nf-L Measurements in Body Fluids
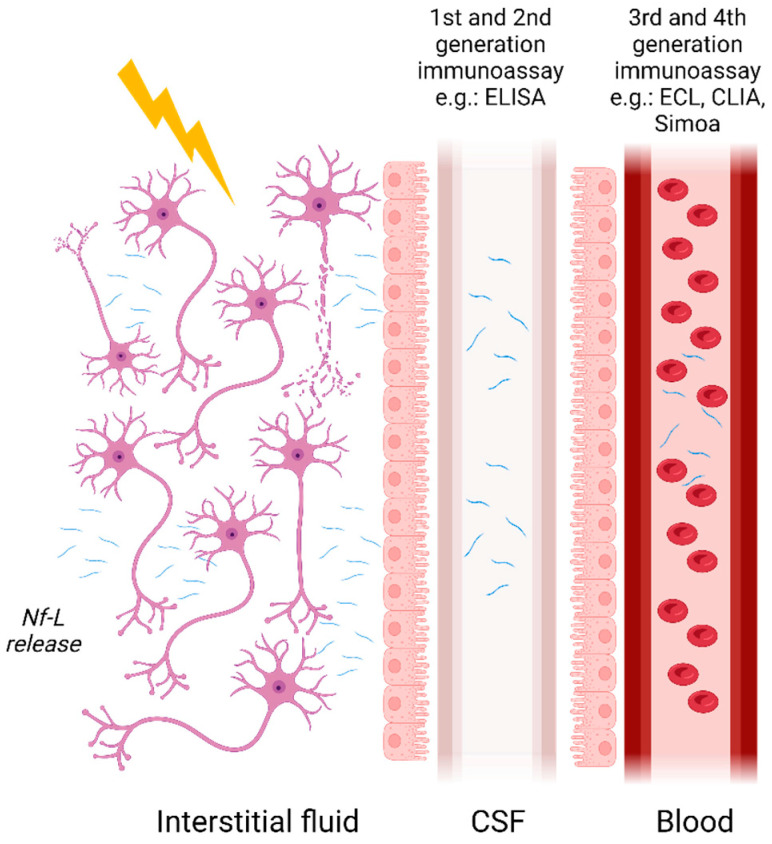
Initially measured using semi-quantitative methods such as immunoblotting, Nf-L concentrations are now measurable in blood and its derivatives owing to the advent of ultrasensitive assays. Nf-Lin human CSF was first measured with ELISA developed by Rosengren et al. in 1996 [3]. Subsequently, more assays have followed such as the commercially available UmanDiagnostic assay for measurement in CSF samples but more recently it has become possible to measure Nf-L in serum with a limit of quantification (LOQ) of 0.8 pg/mL. Other assays developed, such as those described by Gaetani et al. [21] and Das et al. [22], rely on newer ELISA methods with a LOQ of 78 pg/mL [21] and 100 pg/mL [22] in CSF, respectively. Finally, due to the development of ultrasensitive assays such as microfluidic platforms, e.g., Ella (ProteinSimple), ECL assays including Meso Scale Discovery (MSD) from Meso Scale Diagnostic or Attelica Solutions (Siemens), and the Simoa (Quanterix), measurement of Nf-L in blood and its derivatives have become easily achievable with LOQs in the low pg/mL range (Figure 1). Other manufacturers are developing automated assays for the quantification of Nf-L, such as Roche on the Cobas platform and Fujirebio on the Lumipulse. The use of non- or minimally invasive sampling methods such as blood collection is now preferred compared with lumbar puncture. The transition from CSF to blood has greatly improved patient well-being and allowed more samples to be obtained more frequently. Indeed, measurement in serum or plasma permits more longitudinal studies thereby improving the utilization of Nf-L for monitoring disease activities and treatment effects [23]. A reasonably good correlation between CSF and blood Nf-L levels has been established in various studies. For instance, Kuhle et al. [24] demonstrated a good correlation with an r2 of 0.88 between CSF and serum Nf-L measurements using the Simoa platform and 0.78 with the MSD. Similar results were obtained for Nf-L in CSF and blood samples from two cohorts using the Simoa platform [10].
The release of Nf-L in biofluids and the available immunoassay methods for its measurement in different matrices. Nf-L enters from the interstitial fluid into CSF and crosses the blood–CSF barrier making its way to the bloodstream.
Despite the emergence of sensitive techniques and a better understanding of Nf-L as a biomarker, some challenges remain to be addressed before fully implementing Nf-L in clinical practice. First of all, there is a lack of standardization for this marker. In the meta-analysis published by Forgrave et al. [25], whilst results correlated large differences in absolute values for Nf-L quantification were observed across the numerous studies reviewed. Therefore, standardization of Nf-L measurement would be valuable for inter-laboratories studies but also for establishing reliable cut-off values for this biomarker. Additionally, identifying which analytical and clinical factors impact Nf-L levels is also important to define those thresholds.
3.2. Analytical Variability
3.2.1. Type of Sample and Sampling Effects
Nf-L values may vary depending on the type of body fluid in which it is measured. Nf-L in CSF is often more concentrated than in blood due to its proximity to the brain. This difference is also present between plasma Nf-L and serum Nf-L. For plasma, the type of collection tube used for sampling has an impact on the total Nf-L levels measured. For example, Ashton et al. [26] measured plasma Nf-L collected in tubes with different additives: lithium-heparin (LiHep), ethylenediaminetetraacetic acid (EDTA), citrate or NF-L in serum. Nf-L values were slightly different between the four types of collection. Serum and LiHep-plasma showed the highest concentrations as compared to EDTA-plasma and citrate-plasma. EDTA-plasma and serum are the most commonly used, nevertheless is not clear yet which one is the most suitable, the selection for one or the other being specific to the disease or clinical center: for some neurodegenerative diseases such as ALS or MS, the serum is preferred (328 publications on PubMed vs. 104 in plasma), whereas, for dementias, plasma is more often used (382 publications vs. 209 in serum). Furthermore, study comparisons are sometimes difficult due to the preferential use of either plasma or serum. Some researchers have therefore established a correlation between EDTA-plasma and serum [27].
3.2.2. Sample Handling and Storage
Sample treatment is also a source of variability as storage temperature, delayed freezing and multiple freeze–thawing cycles can alter the sample and affect the measurement. Nonetheless, good stability for this marker can be achieved after 3–4 freeze–thawing cycles and where freezing is delayed to 24 h at 2–8 °C or room temperature [26,28,29].
4. Metrological Challenges
To ensure clinical implementation, an RMP and CRM should be developed to allow standardization and traceability of results over time and space. Efforts are ongoing to standardize the measurement of Nf-L through various initiatives, e.g., the CSF working group of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Powerful techniques such as mass spectrometry have been employed to develop both CRMs and RMP but also to define the measurand.
4.1. Developing Certified Reference Materials and Reference Measurement Procedures
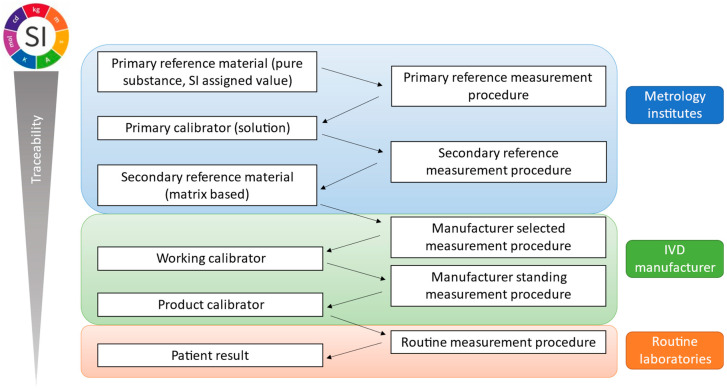
By definition, metrological traceability is the property of a measurement result associated with a reference [32]. This is ensured and documented through a reference measurement system with an unbroken traceability chain as presented in Figure 2 and its principles are described in ISO 17511:2020 [33]. A reference measurement system includes two main components: CRMs or calibrators and reference measurement procedures.
Metrological traceability chain outlined by ISO 17511:2020 indicating elements of the traceability chain across metrology institutes, IVD manufacturers and routine laboratories.
5. Clinical Challenges
Identifying reliable clinical thresholds would be invaluable from the clinical point-of-view to better stratify the different patient groups. It is known that several pathophysiological conditions result in different Nf-L concentrations in body fluids and a better understanding of the clinical background is essential prior to the establishment of cut-off values.
6. Conclusions
This review describes the current limitations of bioassays for Nf-L and their implementation in clinical practice. The major untackled challenges relate to the variability of results and to the numerous clinical and analytical factors affecting the measurement of this biomarker.
Despite the use of sensitive and robust instruments for the quantification of Nf-L in biological fluids, the obtained values vary considerably which can lead to misinterpretation. Standardization of Nf-L measurement would be invaluable to ensure the harmonization of values globally. The development of CRM and RMP, not only in CSF but also in blood, is crucial in the near future to support the diagnostic use of Nf-L in clinics. Moreover, the factors influencing Nf-L levels outlined in this review are important and must be considered carefully. Besides the urgent need for measurement standardization, the establishment of cut-off values will be key for accurate use of Nf-L for diagnosis, prognosis and patient follow-up.