Today, I review, link to, and excerpt from The Cribsiders‘ #102: RSV Immunizations*.
*Creech B, Masur S, Williams P, Chiu C, Berk J. “102: RSV Immunizations”. The Cribsiders Pediatric Podcast. https:/www.thecribsiders.com/ January 31, 2024.
All that follows is from the above resource.
Summary:
The Curbsiders and Cribsiders are back with a very special Med-Peds collaboration on RSV immunizations! Dr. Buddy Creech, Pediatric Infectious Disease at Vanderbilt, walks us through these breakthrough “vaccines” for both adults and kids. He teaches us about their mechanisms of action, eligibility criteria, and why it’s been so challenging to find doses this season. Get ready for your next shot of fun!
RSV Immunization Show Notes and Pearls
RSV Demographics and Public Health
- Respiratory Syncytial Virus (RSV) shows a U-shaped curve in morbidity and mortality, impacting both infants (<9 months) and older individuals (60yo+).
- RSV is the number one cause of hospitalization among pediatric patients.
- 6% of hospitalization for lower respiratory tract infections are related to RSV.
- Protecting adult infections may have public health implications in limiting transmission to cause pediatric infections.
Pediatric Immunization
- Synagis (Pavilizumab) previously was only option
- Synagis limitations included short half-life (20d) requiring multiple doses.
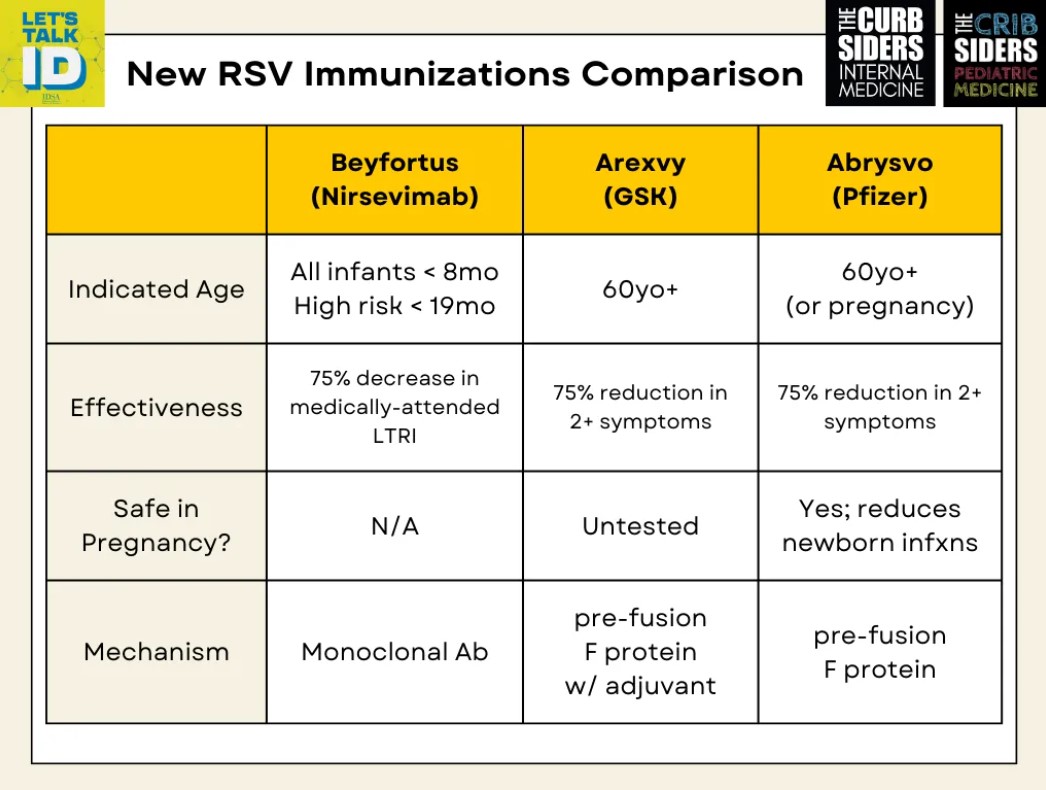
- Beyfortus (Nirsevimab) is new monoclonal antibody that targets the pre-fusion form of the RSV F protein.
- Nirsevimab offers longer protection (5 months) with a single dose.
- It’s now recommended for infants under 8 months and high-risk children up to 19 months.
- Unlike Pavilizumab, Nirsevimab also targets the pre-fusion F protein on the surface of the virus before it docks with the cell.
- Nirsevimab is associated with a 75% decrease in all medically attended lower respiratory tract infections (with a statistically insignificant trend to decrease in hospitalizations by 60%.) – MELODY Trial
- Dosing by weight: <5kg = 50mg; >5kg =100mg
Adult Immunizations
- GSK Arexy vaccine has adjuvant which may make vaccine more potent, and certainly makes side effects more potent.
- Pfizer Abrysvo does not have an adjuvant and has been shown to be safe in pregnancy.
- Both vaccines show ~75% vaccine efficacy in reducing two or more symptoms of RSV in the first three months after immunization
- There was a theoretical concern of giving adjuvant GSK Arexy with the influenza vaccine, but a recent trial showed this to be safe. (Lancet)