These are my three posts on recent Lipid Guidelines:
- 2020 Lipid Guidelines Review – Part 1 – The Curbsiders #191 Lipids Update And Cardiovascular Risk
- 2020 Lipid Guidelines Review – Part 2 – Links And Excerpts From “The 2018 AHA/ACC Guideline on the Managenent of Blood Cholesterol: Executive Summary”
- 2020 Lipid Guidelines Review – Part 3 – Links And Excerpts From “The 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk” [This post]
Today I review, link to, and excerpt from the 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Eur Heart J. 2020 Jan 1;41(1):111-188. doi: 10.1093/eurheartj/ehz455.
Here are excerpts:
4.1.1 Rationale for assessing total cardiovascular disease risk
All current guidelines on the prevention of ASCVD in clinical practice recommend the assessment of total CVD risk. Prevention of ASCVD in a given person should relate to his or her total CV risk: the higher the risk, the more intense the action should be.
Many risk assessment systems are available and have been comprehensively reviewed (Supplementary Table 1 in the Supplementary Data). Most guidelines use one of these risk assessment systems.68
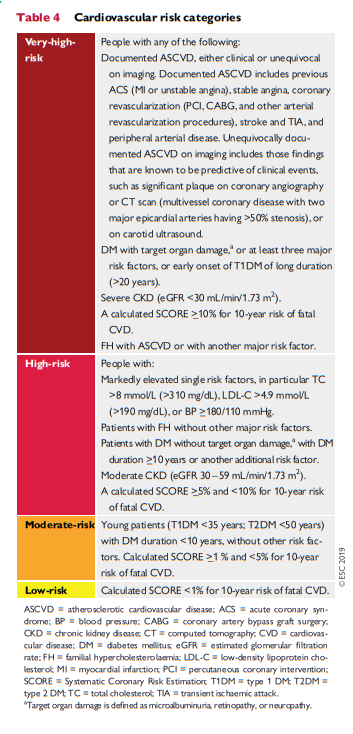
[You will use the following table to upgrade the risk by the risk calculator you are using. This is because no risk calculator can capture all of the risk factors of cardiovascular disease.]
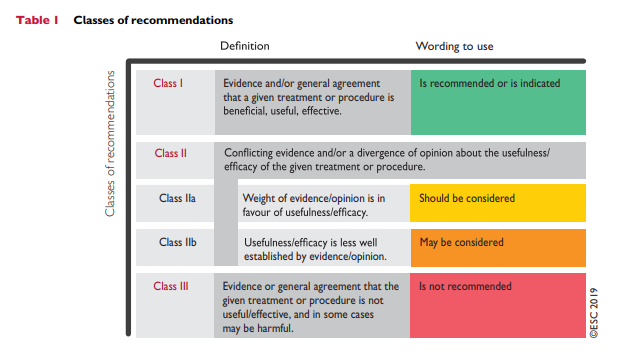
Box 5 Risk estimation: key messages
In apparently healthy persons, CVD risk is most frequently the result of multiple, interacting risk factors. This is the basis for total CV risk estimation and management.
Risk factor screening including the lipid profile should be considered in men >40 years old, and in women >50 years of age or post-menopausal. A risk estimation system such as SCORE can assist in making logical management decisions, and may help to avoid both under- and overtreatment.
Certain individuals declare themselves to be at high or very high CVD risk without needing risk scoring, and all risk factors require immediate attention. This is true for patients with documented CVD, older individuals with longstanding DM, familial hypercholesterolaemia, chronic kidney disease, carotid
or femoral plaques, coronary artery calcium score >100, or extreme Lp(a) elevation.All risk estimation systems are relatively crude and require attention to qualifying statements.
Additional factors affecting risk can be accommodated in electronic risk estimation systems such as HeartScore (www.heartscore.org). The total risk approach allows flexibility; if optimal control cannot be achieved with one risk factor, trying harder with the other factors can still reduce risk.
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; SCORE = Systematic Coronary Risk Estimation.
4.2.1 Role of non-invasive cardiovascular imaging techniques in the assessment of total cardiovascular disease risk
Non-invasive imaging techniques can detect the presence, estimate the extent, and evaluate the clinical consequences of atherosclerotic vascular damage. Detection of coronary artery calcification with noncontrast computed tomography (CT) gives a good estimate of the atherosclerotic burden and is strongly associated with CV events.18 A recent meta-analysis from the US Preventive Services Task Force summarized the available evidence on the value of non-traditional risk factors for risk prediction, and found that, although there are no randomized trials showing that the use of CAC reduces health outcomes, nevertheless it improves both discrimination and reclassification.19 Assessment of carotid or femoral plaque burden with ultrasound has also been demonstrated to be predictive of CV events, comparable to CAC,2023 while the measurement of the carotid intimamedia thickness is inferior to CAC score and carotid plaque detection.16,24,25
In asymptomatic patients at low or moderate risk who would be
eligible for statin therapy (see Table 5), assessment of ASCVD with imaging may have an impact on medical treatment, both from the physician’s and the patient’s points of view. Data from the MultiEthnic Study of Atherosclerosis (MESA) showed that 41 – 57% of individuals who would be eligible for statin therapy had a CAC score of zero and the rate of atherosclerotic CVD events in the 10 year follow-up period was low (1.5 – 4.9%).26 In contrast, the rates of ASCVD and coronary heart disease (CHD) events in individuals with a CAC score >100 Agatston were 18.9 and 12.7 per 1000 personyears, respectively.18 Compared with a strategy of treating all patients, the use of CAC score to guide long-term statin therapy has been shown to be cost-effective.27Note that CAC score is often very low in patients younger than 45 years of age with severe familial hypercholesterolaemia (FH), including homozygous FH (HoFH), and has low specificity in this population. [Emphasis Added]
Assessment of coronary luminal stenosis >50% and plaque composition with coronary CT angiography also provides incremental prognostic value over traditional risk stratification models.28 As a result, in asymptomatic individuals with moderate risk, the presence of a CAC score >100 Agatston, and carotid or femoral plaque burden on ultrasonography, may reclassify them to a higher risk category. Therefore, the use of methods to detect these markers should be of interest in that group (see Recommendations for cardiovascular imaging for risk assessment of atherosclerotic cardiovascular disease
below).1416 Overall, CAC score assessment with CT may be considered in individuals at low or moderate risk in whom the respective LDL-C goal is not achieved with lifestyle intervention alone, and pharmacological therapy is an option (see Table 5).The use of imaging techniques to determine the presence and extent of atherosclerotic vascular damage in low-risk individuals not being considered for statin therapy is not justified due to low prognostic yield, and the associated
costs and radiation hazards when measuring CAC score, particularly among low-risk women.29 Of note, CAC score is increased following statin treatment; therefore, the CAC scores of statin-treated patients should be interpreted with caution.
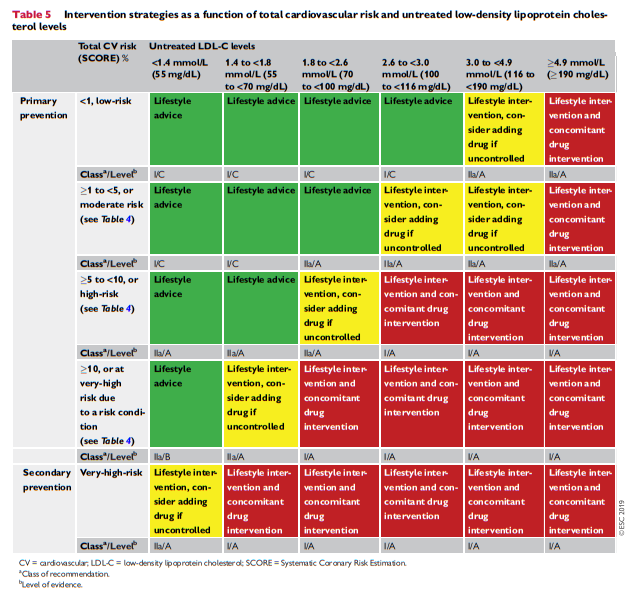
4.2.2 Risk-based intervention strategies
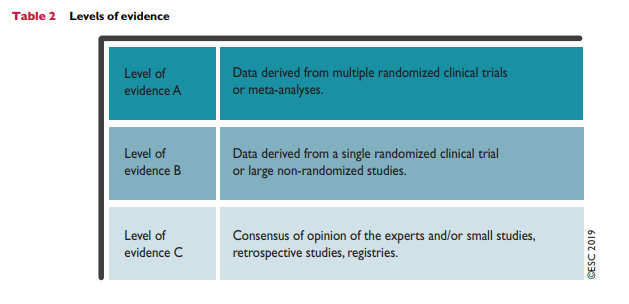
Table 5 presents suggested intervention strategies as a function of total CV risk and LDL-C level. This graded approach is based on evidence from multiple meta-analyses and individual randomized controlled trials (RCTs), which show a consistent and graded reduction in ASCVD risk in response to reductions in TC and LDL-C levels (see Recommendations for cardiovascular disease risk estimation below).31-41
5 Lipids and lipoproteins
5.1 Biological role of lipids and lipoproteins
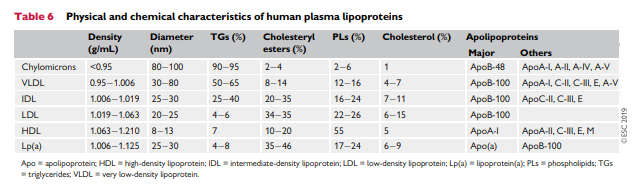
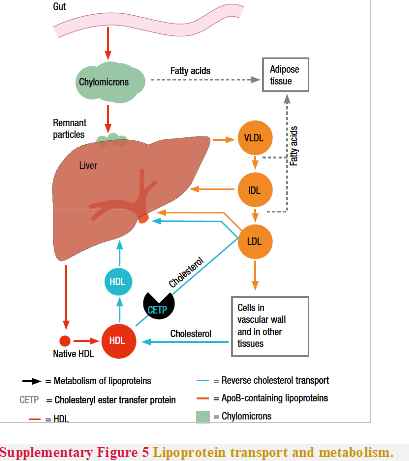
Lipoproteins in plasma transport lipids to tissues for energy utilization, lipid deposition, steroid hormone production, and bile acid formation. Lipoproteins consist of esterified and unesterified cholesterol, TGs, and phospholipids and protein components named apolipoproteins that act as structural components, ligands for cellular receptor binding, and enzyme activators or inhibitors. There are six major lipoproteins in blood: chylomicrons, very lowdensity lipoprotein (VLDL), intermediate-density lipoprotein (IDL), LDL; Lp(a), and HDL (Table 6 and Supplementary Figure 5).
5.2 Role of lipids and lipoproteins in the pathophysiology of atherosclerosis
pp. 127 + 128 of the PDF.
5.3 Evidence for the causal effects of lipids and lipoproteins on the risk of atherosclerotic cardiovascular disease
pp. 128 + 129 of the PDF.
5.3.1 Low-density lipoprotein cholesterol and risk of
atherosclerosisp. 128 of the PDF.
5.3.2 Triglyceride-rich lipoproteins and risk of atherosclerosis
pp. 128 + 129 of the PDF.
5.3.3 High-density lipoprotein cholesterol and risk of
atherosclerosis.p. 129 of the PDF.
The inverse association between plasma HDL-C and the risk of
ASCVD is among the most consistent and reproducible associations in observational epidemiology.45,60In contrast, Mendelian randomization studies do not provide compelling evidence that HDL-C is causally associated with the risk of ASCVD.49,61,62
However, this evidence must be interpreted with caution because most genetic variants associated with HDL-C are also associated with directionally opposite changes in TGs, LDL-C, or both, thus making estimates of the effect of HDL-C on the risk of ASCVD very difficult using the Mendelian randomization study design.
Furthermore, there is no evidence from randomized trials that
therapeutically increasing plasma HDL-C reduces the risk of CV
events.63-67Therefore, there is currently no randomized trial or genetic evidence to suggest that raising plasma HDL-C is likely to reduce the risk of ASCVD events. Whether therapies that alter the function of HDL particles will reduce the risk of ASCVD is unknown.
5.3.4 Lipoprotein(a) and risk of atherosclerosis
p. 128 of the PDF.
Lp(a) is an LDL particle with an Apo(a) moiety covalently bound to its ApoB component.70 It is <70 nm in diameter and can freely flux across the endothelial barrier, where it can become—similarly to LDL—retained within the arterial wall and thus may increase the risk of ASCVD.
Higher plasma Lp(a) concentrations are associated with an
increased risk of ASCVD, but it appears to be a much weaker risk factor for most people than LDL-C.72,73 In contrast, Mendelian randomization studies have consistently demonstrated that lifelong exposure to higher Lp(a) levels is strongly and causally associated with an
increased risk of ASCVD.74,75 While randomized trials evaluating therapies that lower Lp(a) by 20-30% (including niacin and CETP inhibitors*) have not provided evidence that lowering Lp(a) reduces the risk of ASCVD beyond that which would be expected from the observed reduction in ApoB-containing lipoproteins, recent data with PCSK9 inhibitors have suggested a possible role for Lp(a) lowering in reducing CV risk.76
* Trials and Tribulations of CETP Inhibitors [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Circ Res. 2018 Jan 5;122(1):106-112. doi: 10.1161/CIRCRESAHA.117.311978. Epub 2017 Oct 10.
The above article has been cited by 20 PubMed Central Articles.
5.4.2 Lipid measurements
In clinical practice, the concentration of plasma lipoproteins is not usually measured directly but is instead estimated by measuring their cholesterol content. TC in humans is distributed primarily among three major lipoprotein classes: VLDL, LDL, and HDL. Smaller amounts of cholesterol are also contained in two minor lipoprotein classes: IDL and Lp(a).
A standard serum lipid profile measures the concentration of TC and HDL-C, as well as TG. With these values, the LDL-C concentration can be estimated. [Emphasis added]
Plasma LDL-C can be measured directly using enzymatic techniques or preparative ultracentrifugation, but in clinical medicine it is most often calculated using the Friedewald formula:
LDL-C = TC HDL-C (TG/2.2) in mmol/L
or
LDL-C = TC HDL-C (TG/5) in mg/dL
Although convenient, the Friedewald calculated value of LDL-C
has several well-established limitations: (i) methodological errors may accumulate since the formula necessitates three separate analyses of TC, TGs, and HDL-C; and (ii) a constant cholesterol/TG ratio in VLDL is assumed.With high TG values (>4.5 mmol/L or >400 mg/
dL) the formula cannot be used. This should especially be considered in non-fasting samples.For the general population, calculated LDL-C and direct LDL-C
show very strong correlations.8083 However, calculated LDL-C has been found to underestimate LDL-C at concentrations of TG >/= 2 mmol/L (177 mg/dL).81,82 Equally, at very low levels of LDL-C, calculated LDL-C may be misleading, especially in the presence of high TG.81,8486 . . . It is important to note that direct LDL-C measurements also have limitations, including systematic bias and inaccuracy in patients with dyslipidaemia, especially for high TG levels.8890As an alternative calculated LDL-C, non-HDL-C can be calculated as TC HDL-C and is a measure of the TC carried by all atherogenic ApoB-containing lipoproteins, including TG-rich particles in VLDL and their remnants.100
Several methods for the determination of Lp(a) are available. The complex molecular structure of Lp(a) and the variation in size of Apo(a) has been a challenge in the development of analytical methods for Lp(a). Available methods are, to a varying degree, influenced by the Apo(a) isoform.91
Furthermore, the concentration of Lp(a) is
reported as either a molar concentration (nmol/L) or as a mass (mg/ dL) by the various assays, and conversion between molar and mass concentrations has been found to be both size- and concentration dependent.9193Therefore, standardization between assays is [of Lp(a)] of no clinical significance. Indeed, a number of guidelines recommend
non-fasting sampling.100,102,103For general risk screening, non-fasting samples seem to have at
least the same prognostic value as fasting samples.104 The practical advantages of non-fasting samples, including better patient acceptability, outweigh the potential imprecision in some patients, although the determination of some key analytes, such as fasting glucose, may be compromised.Furthermore, even if non-fasting sampling can be used
in most cases, in patients with metabolic syndrome (MetS), DM, or hypertriglyceridaemia (HTG), calculated LDL-C should be interpreted with caution.