In this post, I link to and excerpt from Laboratory Detection and Initial Diagnosis of Monoclonal Gammopathies: Guideline From the College of American Pathologists in Collaboration With the American Association for Clinical Chemistry and the American Society for Clinical Pathology [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Arch Pathol Lab Med. 2022 May 1;146(5):575-590. doi: 10.5858/arpa.2020-0794-CP.
All that follows is from the above resource.
Abstract
Context.—: The process for identifying patients with monoclonal gammopathies is complex. Initial detection of a monoclonal immunoglobulin protein (M protein) in the serum or urine often requires compilation of analytical data from several areas of the laboratory. The detection of M proteins depends on adequacy of the sample provided, available clinical information, and the laboratory tests used.
Objective.—: To develop an evidence-based guideline for the initial laboratory detection of M proteins.
Design.—: To develop evidence-based recommendations, the College of American Pathologists convened a panel of experts in the diagnosis and treatment of monoclonal gammopathies and the laboratory procedures used for the initial detection of M proteins. The panel conducted a systematic literature review to address key questions. Using the Grading of Recommendations Assessment, Development, and Evaluation approach, recommendations were created based on the available evidence, strength of that evidence, and key judgements as defined in the Grading of Recommendations Assessment, Development, and Evaluation Evidence to Decision framework.
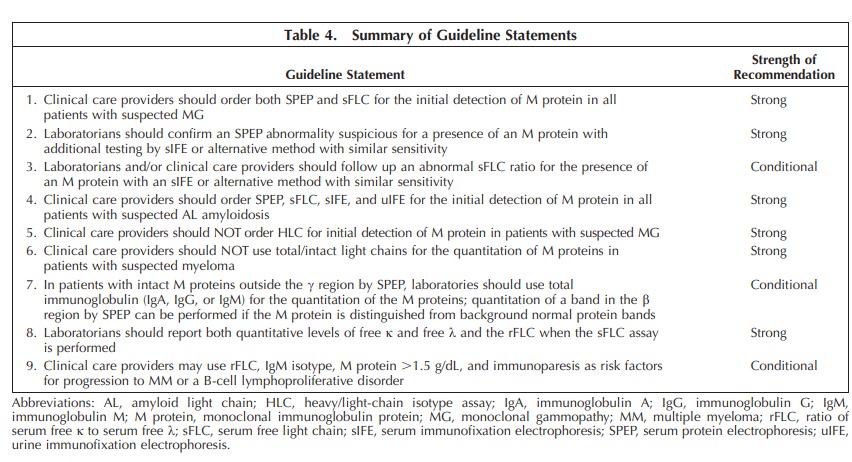
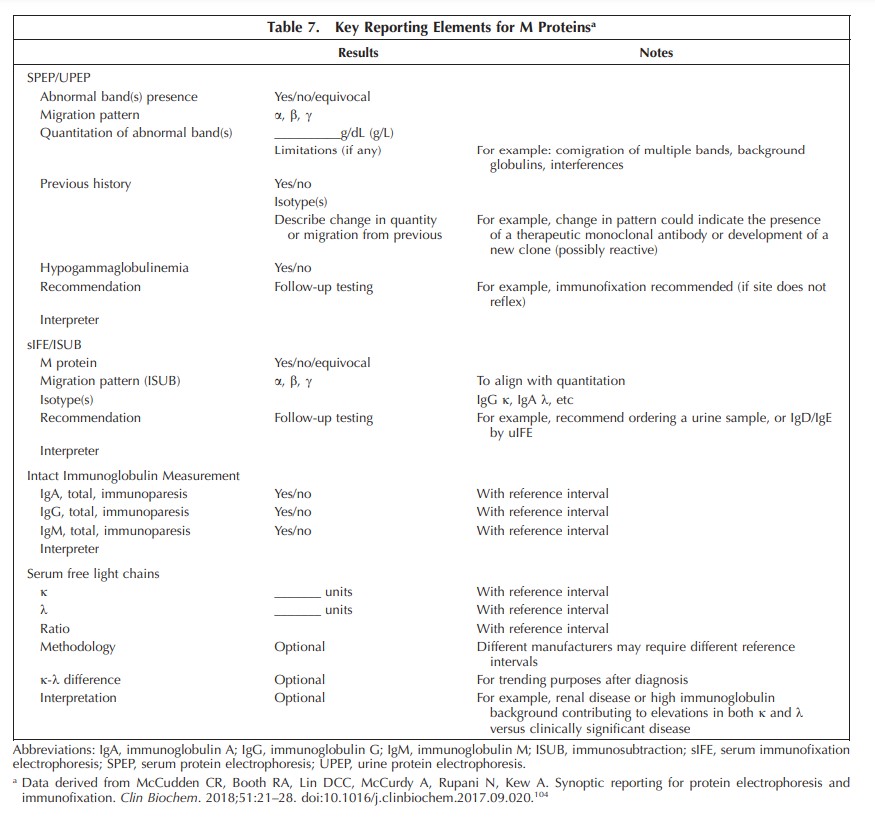
Results.—: Nine guideline statements were established to optimize sample selection and testing for the initial detection and quantitative measurement of M proteins used to diagnose monoclonal gammopathies.
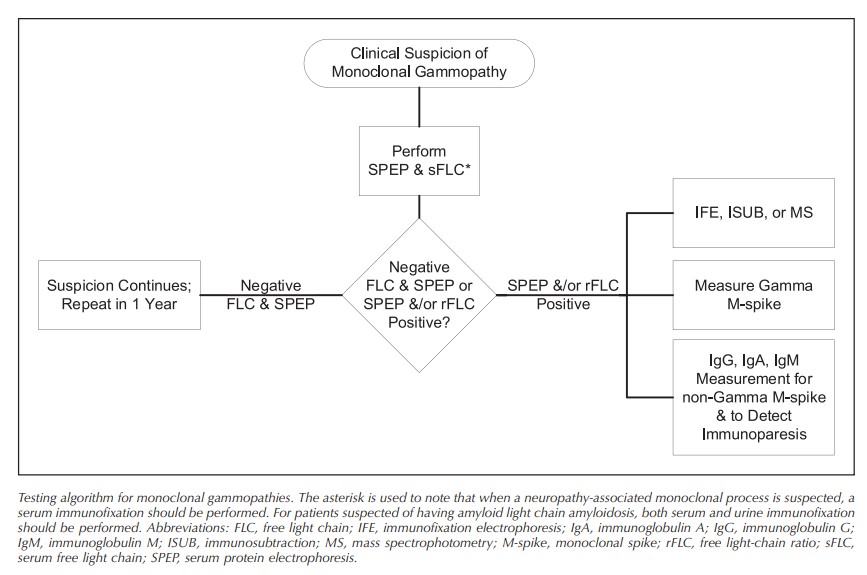
Conclusions.—: This guideline was constructed to harmonize and strengthen the initial detection of an M protein in patients displaying symptoms or laboratory features of a monoclonal gammopathy. It endorses more comprehensive initial testing when there is suspicion of amyloid light chain amyloidosis or neuropathies, such as POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) syndrome, associated with an M protein.
CONCLUSIONS
A comprehensive survey demonstration of heterogeneity of clinical laboratory testing for identifying M proteins indicated likely suboptimal detection of treatable MGs in current practice. Based on a systematic review of theliterature, the EP concluded that sFLC is an essential feature to include for the initial detection of an M protein. For conditions with relatively small quantities of M proteins, IFE of serum and occasionally urine is also needed. This guideline provides 9 statements and background details to assist laboratory implementation of techniques to improve and harmonize the initial detection of patients with MGs.
Guideline Revision
This guideline will be reviewed every 4 years from publication, or earlier in the event of publication of substantive and high-quality evidence that could potentially alter the original guideline recommendations. If necessary, the EP will reconvene to discuss potential changes. When appropriate, the panel will recommend revision of the guideline to the CAP and collaborators for review and approval.