The following are excerpts from Acid-Base Interpretation, updated October 30, 2015 from emedicine.medscape.com:
Interpretation
Respiratory acidosis (acute and chronic)
Respiratory alkalosis (acute and chronic)
Three-step approach
A 3-step approach is used to assess the acid-base disorder: (1) establishment of a primary disturbance, (2) determination of the serum anion gap, and (3) evaluation of compensation.
Establishment of primary disturbance
The pH, obtained from arterial blood gas (ABG)*, should be the first value analyzed upon suspicion of an acid-base disorder. A low blood pH is referred to as an acidemia; acidemia results when an acid-generating process, known as an acidosis, creates an excess of H+ ions. Similarly, an alkalemia refers to elevated blood pH when an alkalosis is present.
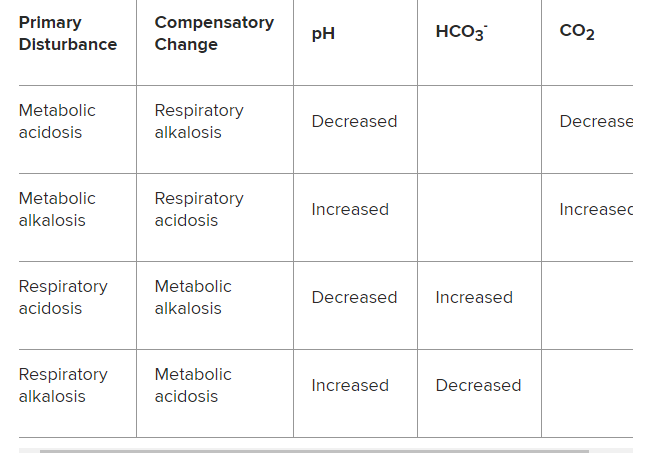
*Please see Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department [PubMed Abstract]. Emerg Med J. 2001 Sep;18(5):340-2. And Can VBG analysis replace ABG analysis in emergency care? [PubMed Abstract]. Emerg Med J. 2016 Feb;33(2):152-4. doi: 10.1136/emermed-2014-204326. Epub 2014 Dec 31The changes in pH, bicarbonate (HCO3–), and carbon dioxide (CO2) expected to be seen in the primary processes are as follows:
Metabolic acidosis: Decreased pH, decreased HCO3–
Metabolic alkalosis: Increased pH, increased HCO3–
Respiratory acidosis: Decreased pH, increased CO2
Respiratory alkalosis: Increased pH, decreased CO2
Serum anion gap determination
When a metabolic acidosis is found, the anion gap should be calculated. The anion gap represents the “unmeasured” anions in the blood, which are formed from organic acids that have dissociated in blood. Unmeasured refers to the fact that these anions are not reported in a standard metabolic panel or ABG but are contributing to the acidosis.
Serum anion gap = Na – (Cl + HCO3)
Some clinicians may also include the serum potassium value in the formula, during which the normal range increases by approximately 4 mEq/L, as follows:
Serum anion gap = (Na + K) – (Cl + HCO3)
The newer autoanalyzers measure a higher serum chloride concentration, which lowers the normal range of the anion gap to 3-9 mEq/L (previously 7-13 mEq/L). Because these machines are not yet universal, it is essential to interpret individual results in the context of the laboratory’s reference range.
Evaluation of compensation
The degree of compensation should be assessed to see if the patient is responding appropriately to their altered pH.
Metabolic acidosis is divided into anion gap metabolic acidosis and non–anion gap metabolic acidosis; these two categories have different etiologies and treatments.
In patients with metabolic acidosis, it is important to determine if respiratory compensation is adequate or if the patient has a concurrent respiratory acidosis or alkalosis. Winter formula is used to determine if the change in PCO2 is appropriate.
Winter formula: Expected pCO2 = 1.5 (HCO3 -) + 8 ± 2
If a patient’s pCO2 is higher than the range expected from Winter formula, a concomitant respiratory acidosis is present. If a patient’s pCO2 is lower than expected, a respiratory alkalosis is also present.
Metabolic alkalosis most commonly results from either diuretic use or gastrointestinal losses, such as in vomiting. If the etiology of the alkalosis is unclear from the examination, a urinary chloride concentration may be measured. Gastrointestinal losses are noted to have low urinary chloride levels (< 20 mEq/L), while patients currently on diuretic therapy present with high urinary chloride levels (>20 mEq/L). A complete list of differential diagnoses and workup may be found at metabolic alkalosis.
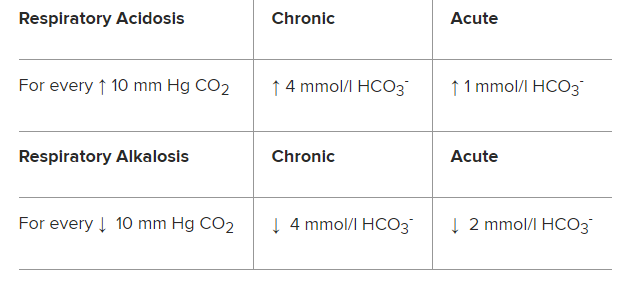
Respiratory acidosis and respiratory alkalosis can be assessed further to determine if the condition is acute or chronic; chronic changes in CO2 allow the renal compensatory mechanism more time to adjust bicarbonate and pH accordingly.
Table 1. Indicators of Chronic and Acute Respiratory Acidosis and Respiratory Alkalosis:
Table 2. Compensatory Acid-Base Disorders Characterized by Changes in pH, HCO3-, and CO2:
Mixed disorders
Compensatory changes do not entirely correct the primary acid-base disorder. If an individual has a primary acidosis, his or her compensatory mechanism will not normalize the pH.
An additional acid-base disorder (ie, a mixed acid-base disorder is present) is suggested by the presence of inappropriate compensation. A normal pH in the setting of abnormal bicarbonate and CO2 levels suggests a mixed acid-base disorder.
The changes in pH in mixed acid-base disturbances are as follows:
Metabolic acidosis: Normal or significantly decreased pH
Metabolic alkalosis: Normal or significantly increased pH
Respiratory acidosis: Normal or significantly decreased pH
Respiratory alkalosis: Normal or significantly increased pH
The mixed disorders include combinations of metabolic disorders, such as vomiting-induced metabolic alkalosis with hypovolemia-induced lactic acidosis; combinations of respiratory disorders, such as COPD related respiratory acidosis with simultaneous opioid-induced hypoventilation; or mixed metabolic and respiratory disorders, such as metabolic acidosis and respiratory alkalosis in salicylate intoxication, among numerous others.
Frequently, the patient’s clinical presentation suggests the cause of the disturbance, and other laboratory values (eg, urine drug screen, serum metabolic panel, serum osmolarity) are used to guide interpretation of the acid-base disturbance.