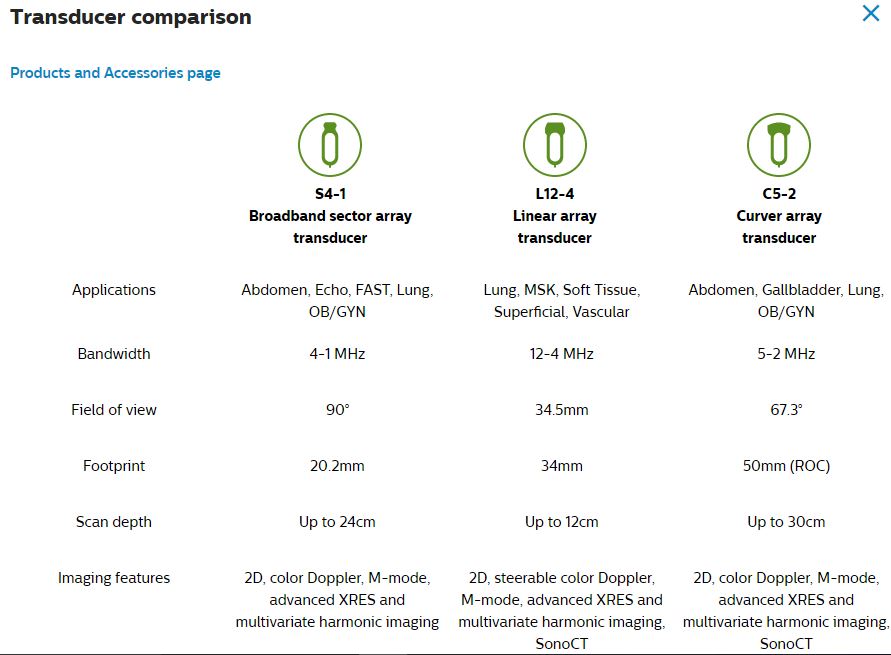
The Phillips Lumify system is a set of three ultrasound probes which can be plugged in to a smart phone Android device. The smart phone device has the Phillips Lumify software downloaded and installed. Thus point of care ultrasound can be available to every clinician. In this post an article on Lumify quality assurance issues is excerpted.
Here are the three probes available for Lumify:
Just like any ultrasound scanner quality assurance for Lumify probes and software is critical. The article below discusses QA for the Lumify device.
The following are excerpts from Diagnostic Ultrasound, in Your Pocket
Published on March 24, 2016 published by 24×7 Magazine:
Diagnostic ultrasound devices, like many technologically intensive products, have undergone a dramatic change over the last 25 years. From a barely movable 500-pound cart-based platform complete with a top-mounted cathode ray tube monitor, to a hand-held “smart device” with either an attached or wireless probe, the physical nature and intended use of ultrasound devices have transformed dramatically since 1990.
The spread of ultrasound technology into point-of-care devices such as the Philips Lumify has brought cost-effective, high-quality imaging and color flow to virtually all points of the globe. But with this major technological change comes rethinking how to properly care for and test the performance of smartphone-based ultrasound devices.
Ultrasound systems have become software-centric with more generic control hardware. However, the probes themselves have become increasingly complex as front-end electronics like the beamformer (previously located in the ultrasound mainframe) have migrated to the probe.
Given that all the electronics necessary to drive a probe from a smartphone reside within the probe itself, it’s important to briefly review how transducers are constructed and how they work to set the stage for the testing and servicing discussion that follows.
Transducer Construction
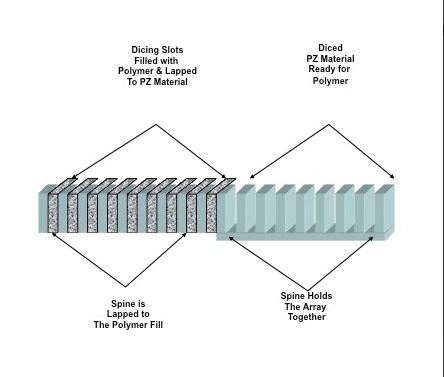
The vast majority of contemporary commercially available composite multielement transducers (CMET) are composed of: 1) a piezoelectric (PZ) material that provides the necessary transduction of mechanical to electrical energy and vice versa, and 2) a supporting polymer that isolates the element while shaping the mechanical and electrical properties of the PZ elements. The physical distribution of PZ and polymer makes the array and specifies its acoustic behavior. The actual array construction is based upon well established “dicing and filling” techniques used in transducer manufacturing.
Array construction begins with a block of PZ material that is “diced” to form PZ elements held by a common spine. The diced regions are then filled with polymer, as seen in Figure 1. By lapping the array “front” and “back” to remove polymer on the face and the PZ spine on the back, the manufacturer sets the array center operating frequency and sets the final configuration. The distribution of PZ and polymer provides a specific pattern of connectivity and coupling.
Connectivity refers to the continuity of each material along each of the three axes. Specific dicing and filling can produce an array that looks like a PZ-polymer sandwich (common to many current linear, convex, and phased arrays), or an array of PZ elements where each is surrounded by polymer (found in 1.5D and 2D arrays).
Coupling refers to the ability to transfer ultrasound out of an array and into the patient’s tissues and the reverse when echoes return. Acoustic coupling improves when quarter-wave layers of material with intermediate acoustic impedance values are bonded to the array face. Both connectivity and coupling can currently be tested on many ultrasound probes using commercially available probe testing devices. Multiple peer-reviewed papers have shown that as few as two dead elements in a CMET transducer can compromise the clinical efficacy of an ultrasound study, reinforcing the need to test transducers on a regular basis.1-3 [Emphasis Added]
Just as in earlier cart-based ultrasound systems, the transducer on the smartphone-based system creates a focused beam. By using phasing controls, the transducer moves that beam in space while dynamically focusing it at various ranges to form a more uniform B-mode image frame. The rules of focusing, such as Huygens’ principle and others, are all managed by electronic controls within the probe housing. These acoustic parameters are beyond the scope of this article, but a white paper is available to those readers who wish for more detailed information.4 Suffice to say that all of these parameters must be well defined, designed, and controlled in order to produce a diagnostic quality image and protect the smart device.
What the ultrasound manufacturer actually sells or rents to the clinical end user is the ultrasound software and a “smart” probe. These companies do not provide the Android device. The smartphone/tablet, via the micro USB port, supplies the probe with power to operate. The downloaded ultrasound software enables patient data entry, ultrasound display, and examination storage. Control signals operate the electronics inside the probe and export the results of the ultrasound examination from the smartphone to the cloud.
Testing probes on these new mobile devices requires the same diligence necessary for probes on cart-based systems, since the intended use and expected outcome of both devices is to create a clinically efficacious image. To that end, benchmarking the clinical performance of a new device will establish a threshold against which periodic quality assurance (QA) testing can be measured. [Emphasis Added]
Although the diffusion of ultrasound technology to commercial off-the-shelf devices will continue to grow rapidly, they are still very basic modality devices with a limited feature set. The more eclectic yet important modalities such as 3D volumetric imaging, shear wave elastography, and strain gauge imaging—all requiring significant processing capacity—remain the domain of cart-based ultrasound systems, and will for the foreseeable future.
High-end, cart-based systems more frequently use complex 2D matrix array transducers for various clinical applications to view the anatomy in a volumetric manner. Although complex, these probes can (and should) be tested and performance-verified on a regular basis by the hospital HTM personnel.
The diffusion of ultrasound technology into mobile devices such as smartphones increases the availability of affordable, quality ultrasound imaging performance for point-of-care applications on a global, and often underserved, basis. Ultrasound probes, too, will continue to get smarter and less expensive as new piezo materials replace current 1-3 composite arrays.
As compelling as this new paradigm is, we must always keep in mind that these mobile devices are transformed into finished medical devices that must be properly maintained to ensure both the quality of clinical images and the safety of the device. By understanding the particulars of any given probe design, as well as documenting the operational quirks of the mobile device being used, we can develop and implement a rational QA and preventive maintenance program.
The shift from hardware-centric to software-centric systems means retooling not only our test device arsenal, but our thinking and equipment maintenance strategies.
Wayne Moore is CEO of Acertara Acoustic Laboratories. For more information, contact chief editor Jenny Lower at jlower@allied360.com.
References
1. The Effect of Dead Elements on the Accuracy of Doppler Ultrasound Measurements, Jaromir Vachutka, Ladislav Dolezal, et al. Ultrasonic Imaging 2014, Vol 36(I) 18-34, Sage Publishing.
2. High Incidence of Defective Ultrasound Transducers in Use in Routine Clinical Practice, Martensson M, Olsson, M, et al. European Journal of Echocardiography, 2009;10:389-94.
3. The Methods and Effects of Transducer Degradation on Image Quality and the Clinical Efficacy of Diagnostic Sonography, Weigang B, Moore GW, et al. Journal of Diagnostic Medical Sonography 2003; 19: 3-13
4. The Silent Revolution: Catching Up with the Contemporary Composite Transducer, Raymond Powis, PhD, FAIUM and G. Wayne Moore, B.Sc., MA, FASE, Acertara Acoustic Laboratories.
5. Mobile Medical Applications, Guidance for Industry, Center for Devices and Radiological Health, US Food and Drug Administration, Document issued on Feb 9, 2015. https://www.fda.gov/downloads/MedicalDevices/…/UCM263366.pdf
6. https://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm435537.htm, US Food and Drug Administration.