In this post, I link to and excerpt from American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD) [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Endocr Pract. 2022 May;28(5):528-562.
All that follows is from the above resource.
Abstract
Objective
To provide evidence-based recommendations regarding the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) to endocrinologists, primary care clinicians, health care professionals, and other stakeholders.Methods
The American Association of Clinical Endocrinology conducted literature searches for relevant articles published from January 1, 2010, to November 15, 2021. A task force of medical experts developed evidence-based guideline recommendations based on a review of clinical evidence, expertise, and informal consensus, according to established American Association of Clinical Endocrinology protocol for guideline development.Recommendation Summary
This guideline includes 34 evidence-based clinical practice recommendations for the diagnosis and management of persons with NAFLD and/or NASH and contains 385 citations that inform the evidence base.Conclusion
NAFLD is a major public health problem that will only worsen in the future, as it is closely linked to the epidemics of obesity and type 2 diabetes mellitus. Given this link, endocrinologists and primary care physicians are in an ideal position to identify persons at risk on to prevent the development of cirrhosis and comorbidities. While no U.S. Food and Drug Administration-approved medications to treat NAFLD are currently available, management can include lifestyle changes that promote an energy deficit leading to weight loss; consideration of weight loss medications, particularly glucagon-like peptide-1 receptor agonists; and bariatric surgery, for persons who have obesity, as well as some diabetes medications, such as pioglitazone and glucagon-like peptide-1 receptor agonists, for those with type 2 diabetes mellitus and NASH. Management should also promote cardiometabolic health and reduce the increased cardiovascular risk associated with this complex disease.Key words
Lay Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease affecting 25% of the global population. Despite the sizable and growing prevalence, disease awareness remains limited with <5% of persons with NAFLD being aware of their disease compared with 38% of persons with viral hepatitis. Twelve to 14% of persons with NAFLD have a more aggressive form known as nonalcoholic steatohepatitis (NASH), which can progress to advanced liver fibrosis, cirrhosis, or liver cancer. The risk of NASH is two- to threefold higher in persons with obesity and/or type 2 diabetes mellitus. NASH is among the top causes of liver cancer and the second most common indication for liver transplantation in the United States after hepatitis C. NAFLD is diagnosed by abnormal liver test results (although liver test results may be normal) and imaging studies, not related to excess alcohol use or other causes of liver disease. NASH is diagnosed by a liver biopsy; however, specialized blood tests and imaging can determine the risk of significant fibrosis. NAFLD is associated with cardiometabolic disorders: (1) obesity, (2) insulin resistance, (3) type 2 diabetes mellitus, (4) high blood pressure, and (5) atherogenic dyslipidemia, all of which increase the risk of a heart attack or stroke, the most common cause of death. The primary treatment of NAFLD is weight loss with a low-calorie diet; restriction of saturated fat, starch, and sugar; improved eating patterns (eg, Mediterranean diet and minimally processed whole foods); and exercise. Cardiometabolic benefit and reduction of liver fat can be observed with >5% weight loss. More weight loss provides increased benefits and may reverse steatohepatitis or liver fibrosis (≥10% weight loss). There are no U.S. Food and Drug Administration-approved medications for the treatment of NAFLD; however, some diabetes and antiobesity medications can be beneficial. Bariatric surgery is also effective for weight loss and reducing liver fat in persons with severe obesity.
Abbreviations
AACE, American Association of Clinical Endocrinology; AASLD, American Association for the Study of Liver Diseases; ABCD, adiposity-based chronic disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; aHR, adjusted hazard ratio; BEL, best evidence level; BMI, body mass index; CAP, controlled attenuation parameter; CI, confidence interval; CKD, chronic kidney disease; CMD, cardiometabolic disease; CPG, Clinical Practice Guidelines; CV, cardiovascular; CVD, cardiovascular disease; EBMT, Endoscopic bariatric and metabolic therapy; ELF, enhanced liver fibrosis; ESG, endoscopic sleeve gastroplasty; FDA, U.S. Food and Drug Administration; FIB-4, fibrosis-4 index; GH, growth hormone; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HCC, hepatocellular carcinoma; HR, hazard ratio; 1H-MRS, proton magnetic resonance spectroscopy; IGB, intragastric balloon; IHTG, intrahepatic triglyceride; IR, insulin resistance; LSM, liver stiffness measurement; MACE, major adverse cardiovascular event; MetS, metabolic syndrome; MRE, magnetic resonance elastography; MRI, magnetic resonance imaging; MRI-PDFF, magnetic resonance imaging-proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, nonalcoholic steatohepatitis; NFS, NAFLD fibrosis score; NPV, negative predictive value; OR, odds ratio; PCOS, polycystic ovary syndrome; PCP, primary care physician; PNPLA3, patatin-like phospholipase domain-containing 3; PPAR, peroxisome proliferator-activated receptor; PPV, positive predictive value; pSWE, point shear wave elastography; RCT, randomized controlled trial; RYGB, Roux-en-Y gastric bypass; SGLT2, sodium-glucose cotransporter 2; SWE, shear wave elastography; TBW, total body weight; TE, transient elastography; T1D, type 1 diabetes mellitus; T2D, type 2 diabetes mellitus; US, ultrasonography; 2DSWE, 2-dimensional shear wave elastography; VCTE, vibration-controlled transient elastography. Structure of Clinical Practice Guideline
1. Introduction
- Epidemiology of Adult and Pediatric NAFLD
- Purpose
- Scope
- Limitations of the Literature
2. Methods
3. Summary of Recommendations: summary list of all recommendations developed for this clinical practice guideline
4. Recommendations With Evidence Base
- Recommendation
- Recommendation Grade, Strength of Evidence Grade, and Best Evidence Level
- Evidence Base: summary of clinical background and highlighted studies that best support the recommendation
Purpose
Given the high prevalence of NAFLD in clinical endocrinology
and primary care practice and the paucity of guidelines that address the metabolic and endocrinologic perspectives, little guidance is available for frontline practitioners who care for persons with NAFLD, most of whom are undiagnosed. The purpose of this guideline is to provide endocrinology and primary care clinicians with practical evidence-based recommendations for the diagnosis and management of NAFLD.Scope
This guideline addresses key management questions and focuses on the metabolic and endocrinologic aspects of prevention,
diagnosis, treatment, and long-term prognosis for the entire population of persons with NAFLD. Outside the scope of this guideline is an in-depth review of the epidemiology in the general population or inclusion of controversial aspects of NAFLD reserved for the liver specialist. It is meant to provide practical patient-centered guidance for endocrinologists and PCPs who often see populations at high risk of developing NASH (ie, those with obesity, MetS, and/or T2D). It also does not address interventions of a purely investigational nature; it includes only those interventions available to the practicing clinician: (1) lifestyle intervention, (2) bariatric surgery, (3) weight loss and diabetes treatment agents, and (4) any other agent with strong evidence from randomized controlled trials (RCTs) deemed as safe and effective.There are no U.S. Food and Drug Administration (FDA)-approved medications for the treatment of NASH available at the time of publication.
Limitations of the Literature
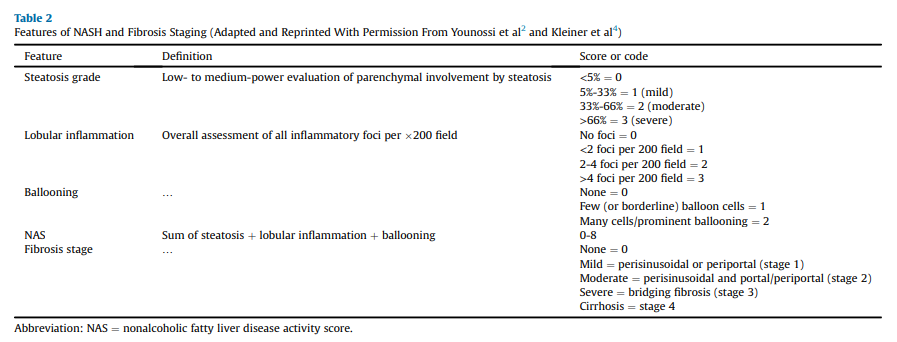
NAFLD has reached epidemic proportions fueled by the increase
in the incidence of obesity and T2D, creating a need for endocrinology and primary care clinicians to become engaged in its early diagnosis and management. Although there is a rapidly growing body of literature, the field still has several knowledge gaps. For instance, while the diagnosis of hepatic steatosis by imaging is rather simple (ie, liver US or MRI-based techniques), there is a lack of robust and well-validated blood tests or imaging studies for the noninvasive diagnosis of nonalcoholic steatohepatitis (NASH). Similar limitations apply to the accurate diagnosis of hepatic fibrosis, with liver biopsy remaining the “gold standard” test for the diagnosis of NASH and for staging the severity of fibrosis. This calls for the stepwise use of noninvasive tests to minimize the need for a
liver biopsy, but still, the vast majority of persons with NASH and advanced fibrosis (stages F2-F4; Table 2) remain undiagnosed in primary care and endocrinology clinics.Recommendations with Evidence Base
Diagnosis of NAFLD in Adults
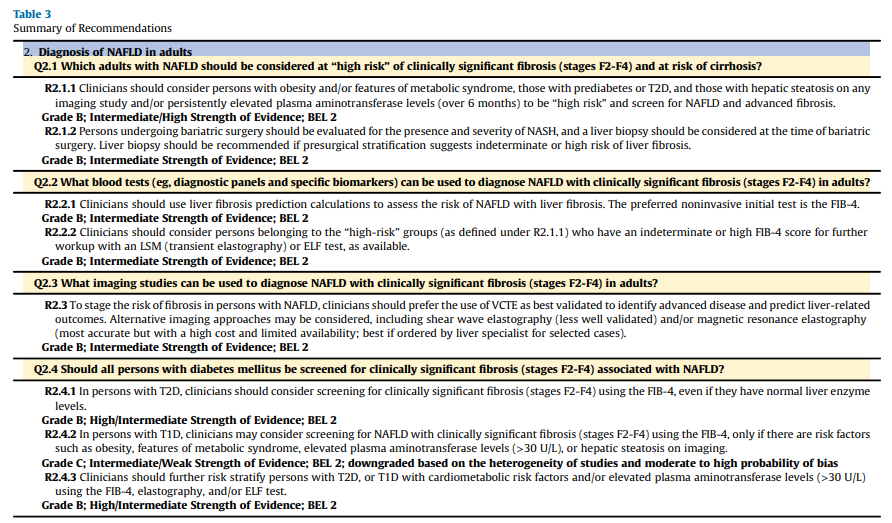
Q2.1 Which Adults With NAFLD Should Be Considered at “High Risk”of Clinically Significant Fibrosis (Stages F2-F4) and at Risk of Cirrhosis?
Recommendation 2.1.1. Clinicians should consider persons with
obesity and/or features of MetS, those with prediabetes or T2D, and those with hepatic steatosis on any imaging study and/or persistently elevated plasma aminotransferase levels (over 6 months) to be “high risk” and screen for NAFLD and advanced fibrosis.Grade B; Intermediate/High Strength of Evidence; best evidence level (BEL) 2
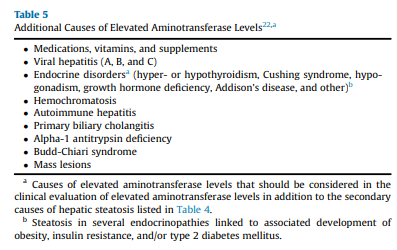
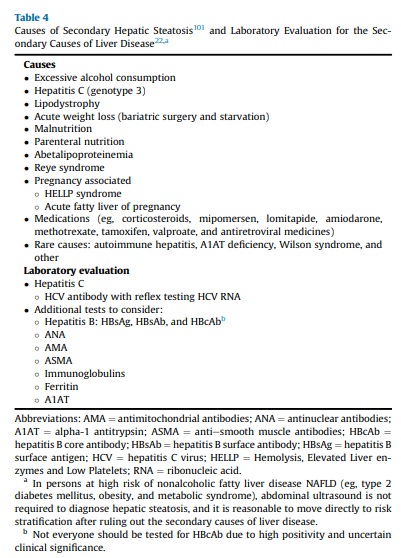
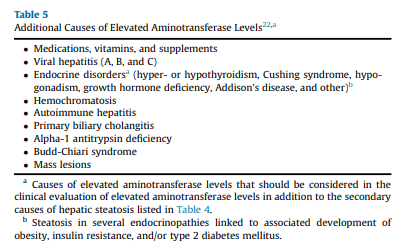
In the past 20 years, it has become evident that persons with T2D have a very high prevalence of NAFLD and associated fibrosis.5,6,9- 11,52,102-104 Additionally, individuals with persistently abnormal aminotransferase levels in the absence of other causes of liver disease (eg, viral hepatitis and excessive alcohol use) are also at high risk of NAFLD and development of hepatic fibrosis (Table 5).105,106 It is important to highlight that a landmark population-based study established that the upper limit of plasma alanine aminotransferase (ALT) should be 30 U/L for men and 19 U/L for women.107 Additional studies havemade the American College ofGastroenterologyconsider
a true normal ALT level to range from 29 to 33 U/L for males and 19 to 25 U/L for females.108 This is because a level above the upper limit of normal, even in a population without identifiable risk factors, is associated with increased liver-related mortality and should be evaluated by clinicians. In this context, it is important to remember that persons with NAFLD and normal aminotransferase levels can still have significant steatohepatitis and develop advanced fibrosis or cryptogenic cirrhosis,30,109 but the presence of high aminotransferase
levels does increase the prevalence of adverse outcomes.106Screening for NAFLD to prevent future cirrhosis is justified based on recent studies indicating a high prevalence of liver fibrosis (12%- 21%) in persons with T2D5,6,9-11,52,102-105,110,111 and the association of liver fibrosis with the future risk of developing complications of cirrhosis, including ascites, renal dysfunction, HCC, hepatic encephalopathy, and bacterial infections, and overall higher mortality.43,47,112 A recent international cohort of 299 individuals with biopsy-proven NASH and compensated cirrhosis, during a median
follow-up of 5 years, found that having T2D increased the risk of
death (adjusted HR [aHR], 4.23; 95% CI, 1.93-9.29) and liver-related outcomes (aHR, 2.03; 95% CI, 1.005-4.11), including HCC (aHR, 5.42; 95% CI, 1.74-16.80),46 by approximately twofold. It is well established that cirrhosis and poor outcomes are much more common in persons with diabetes.54Q2.2 What Blood Tests (eg, Diagnostic Panels and Specific
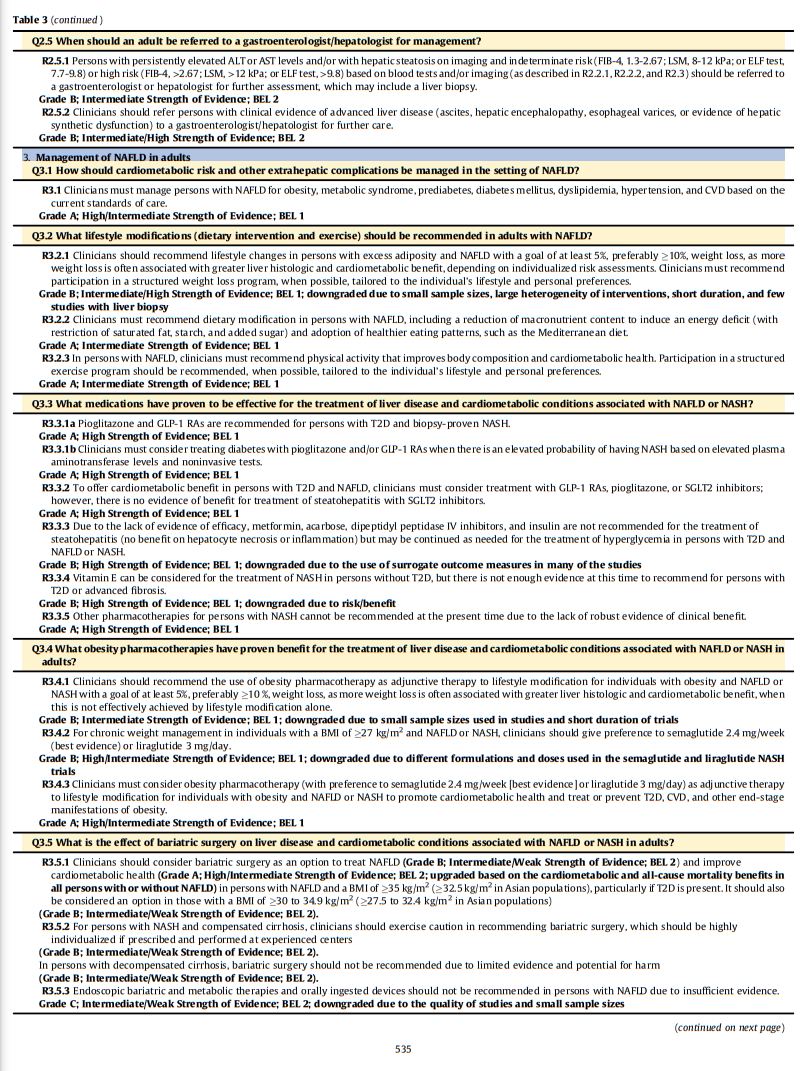
Biomarkers) Can Be Used to Diagnose NAFLD With Clinically
Significant Fibrosis (Stages F2-F4) in Adults?Recommendation 2.2.1. Clinicians should use liver fibrosis prediction calculations to assess the risk of NAFLD with liver fibrosis.
The preferred noninvasive initial test is the fibrosis-4 index (FIB-4).
Grade B; Intermediate Strength of Evidence; BEL 2Evidence Base. Plasma liver aminotransferase levels can be unreliable and normal in many cases of NAFLD128 and should not be used alone for the diagnosis of NAFLD. In a study in persons with T2D, up to 50% had NAFLD despite the so-called “normal” ALT levels (definedas <40 U/L in this study).30 More recent studies have confirmed that the vast majority of persons with NAFLD in primary care or endocrinology clinics, even those with clinically significant fibrosis (≥F2), have a plasma aminotransferase level of <40 U/L.9,10,102,114
Hepatic steatosis can be diagnosed on imaging, including liver US, CAP, computed tomography, or the 2 most accurate and sensitive methods, 1 H-MRS and magnetic resonance imaging-proton density fat fraction (MRI-PDFF).
Most important for endocrinology and primary care clinicians is to calculate liver fibrosis scores for the diagnosis of clinically significant fibrosis, particularly using the FIB-4 (definition shown in Table 1), which has been the most validated among the many tested to this end.43,130,135-141 The FIB-4 has strong validation in its ability to predict changes over time in hepatic fibrosis 135 and allows risk stratification of persons in terms of future liver-related morbidity and mortality, as shown in a population-based prospective survey136 and in a recent meta-analysis of 13 longitudinal studies.137
Of interest, the NAFLD fibrosis score (NFS), a liver score commonly used in hepatology clinics, may overestimate in the primary care setting the prevalence of advanced liver fibrosis in persons with obesity,142 and in particular with T2D11; therefore,it should be avoidedin this setting (noninvasive
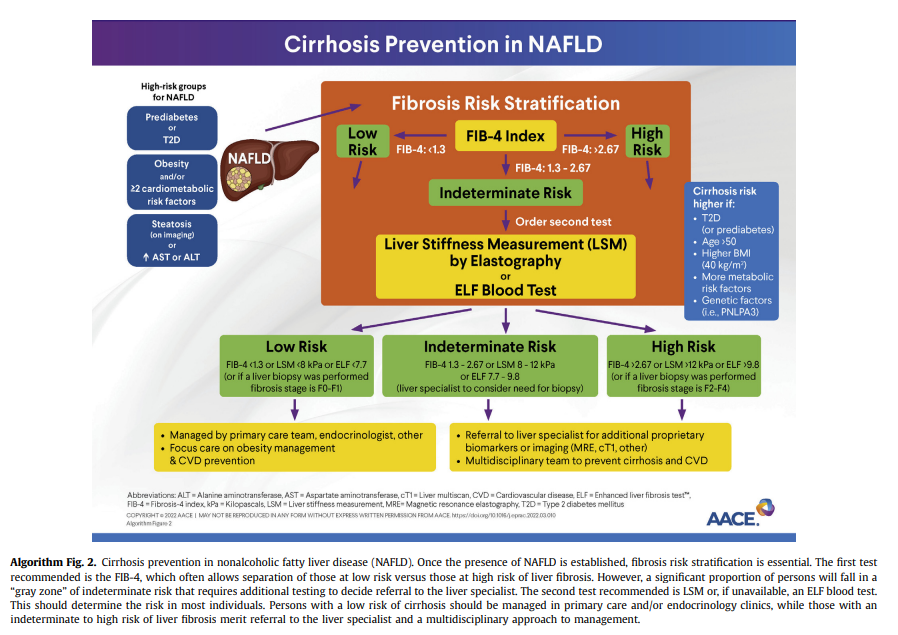
tests and screening tools shown in Table 1). Proprietary biomarkers include the FibroTest, enhanced liver fibrosis (ELF) test,143 propeptide of type III collagen,144-146 NIS4141 and others.130,134,137,147-149Endocrinology and primary care clinicians must be aware of the limitations of blood panels, compared with a liver biopsy (ie, the“gold standard”). Overall, panels for the diagnosis of fibrosis have a good specificity and negative predictive value (NPV) that allow the clinician to rule out advanced fibrosis and use this as a rule-out test.130,134,137,147,148 However, they lack adequate sensitivity and positive predictive value (PPV) to establish the presence of advanced fibrosis; therefore, several individuals fall in the “indeterminate-risk” group (Algorithm Fig. 2).
In this context, a multistep process must be used. For example, the area under the receiver operating characteristic curve for the FIB-4 is 0.78 to 0.80135,147,150,151 but lower for NFS (0.72-0.75), in particular in persons with T2D.147 Of note, their performance is dependent on the population being studied, with a better performance in hepatology clinics where more people have advanced disease than in primary care settings, where the FIB-4 and other tests have been less well characterized.
Recommendation 2.2.2. Clinicians should consider persons
belonging to the “high-risk” groups (as defined under R2.1.1) who have indeterminate or high FIB-4 score for further workup with a liver stiffness measurement (LSM) (TE) or ELF test, as available.
Grade B; Intermediate Strength of Evidence; BEL 2Evidence Base. In endocrine and primary care clinics, the initial step in persons at high risk of having NAFLD (prediabetes, T2D, obesity and/or MetS, or elevated plasma aminotransferase level) is to evaluate their risk of NAFLD. Hepatic steatosis may be assessed by means of simple noninvasive liver steatosis scores (fatty liver index, US fatty liver index, and hepatic steatosis index), although these
diagnostic modalities have inherent limitations.11,115,152 A liver US is not recommended for routine clinical diagnosis.115 Instead, TE is preferred over liver US, where available, as it can quantify liver fat (CAP) and fibrosis (vibration-controlled transient elastography [VCTE]) for risk stratification during the same testing.*
*Please see Vibration-controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Clin Gastroenterol Hepatol. 2019 Jan; 17(1): 156–163.e2.
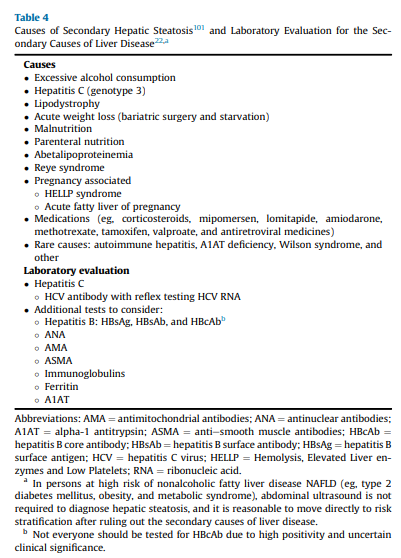
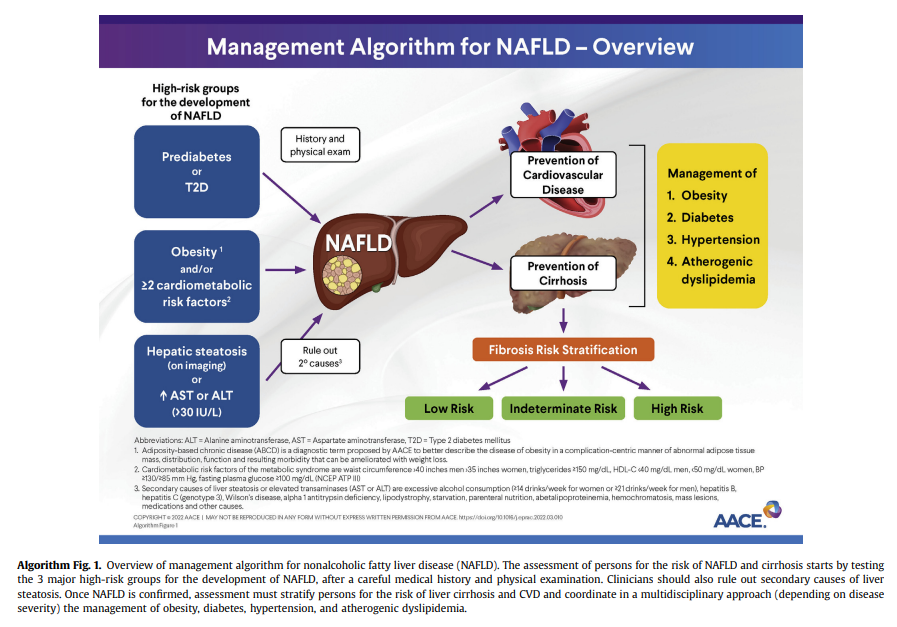
In persons with a high pretest probability of NAFLD, such as the 3 at-risk
groups identified in the diagnostic algorithm (Algorithm Fig. 1), it is reasonable to perform a risk stratification (FIB-4) without the need for a liver US for the diagnosis of hepatic steatosis (ie, in the 3 at-risk groups, the chance of having hepatic steatosis is very high and ≥70%).5,9-11,52,102-104 It is important to perform a complete medical history and routine clinical chemistries that allow clinicians to rule out secondary causes of liver steatosis (Table 4)and elevated plasma aminotransferase levels (Table 5). A thorough workup should be performed to rule out competing causes for steatosis, in addition to excluding significant alcohol consumption.
It is important to assess further for the risk of clinically significant fibrosis (stages F2-F4), which provides prognostic information on the future risk of cirrhosis and can guide treatment strategies, as well as need for referral to a hepatologist/gastroenterologist. A combination of the FIB-4 followed by VCTE (description under Q2.3) seems to be
the best approach. If the FIB-4 score is >1.3, then a second level test,vsuch as VCTE or ELF, should be performed (Algorithm Fig. 2).Using the FIB-4 as a first-line test, followed by VCTE, can help stratify persons in the “indeterminate zone” and greatly reduce the number of referrals to the specialist.130,134,137,147,148,153 Of note, higher cutoffs for the FIB-4, in the range of 1.9 to 2.0 (rather than >1.3), have been suggested with older age (≥ 65 years) to determine advanced fibrosis.154,155
This combination or sequential use of tests yields a higher PPV
in identifying at-risk persons with active NASH and fibrosis. In a study of 968 persons with biopsy-confirmed NAFLD, sequential testing with the FIB-4 or NFS followed by TE in those with an indeterminate score was more accurate than performing either test alone.156 In another cross-sectional study of 3202 persons with bridging fibrosis and compensated cirrhosis, noninvasive tests alone or in combination with imaging (VCTE) reduced the need for a liver biopsy when trying to discriminate advanced fibrosis caused by NASH.150 Persons with high or intermediatefibrosis risk should be referred to hepatology for further evaluation and consideration of a liver biopsy. Liver biopsy remains the “gold standard” for the diagnosis of NASH; however, it should not be used as a screening method to diagnose NAFLD given its multiple caveats: it is invasive, subject to interpretation errors,157 and difficult to apply to large populations. An algorithm to screen for NAFLD and identify those at risk of clinically significant fibrosis has been proposed (Algorithm Fig. 2).Q2.3 What Imaging Studies Can Be Used to Diagnose NAFLD With Clinically Significant Fibrosis (Stages F2-F4) in Adults?
Recommendation 2.3. To stage the risk of fibrosis in persons with NAFLD, clinicians should prefer the use of VCTE as best validated to identify advanced disease and predict liver-related outcomes. Alternative imaging approaches may be considered, including shear wave elastography (SWE) (less well validated) and/or MRE (most accurate but with a high cost and limited availability; best if ordered by a liver specialist for selected cases).
Grade B; Intermediate Strength of Evidence; BEL 2Q2.4 Should All Persons With Diabetes Mellitus Be Screened for
Clinically Significant Fibrosis (Stages F2-F4) Associated With NAFLD?Recommendation 2.4.1. In persons with T2D, clinicians should
consider screening for clinically significant fibrosis (stages F2-F4) using the FIB-4, even if they have normal liver enzyme levels.
Grade B; High/Intermediate Strength of Evidence; BEL 2Recommendation 2.4.2. In persons with T1D, clinicians may
consider screening for NAFLD with clinically significant fibrosis
(stages F2-F4) using the FIB-4, only if there are risk factors such as obesity, features of MetS, elevated plasma aminotransferase levels (>30 U/L), or hepatic steatosis on imaging.
Grade C; Intermediate/Weak Strength of Evidence; BEL 2;
downgraded based on the heterogeneity of studies and moderate to high probability of biasRecommendation 2.4.3. Clinicians should further risk stratify persons with T2D or T1D with cardiometabolic risk factors and/or elevated plasma aminotransferase levels (>30 U/L) using the FIB-4, elastography, and/or ELF test.
Grade B; High/Intermediate Strength of Evidence; BEL 2Q2.5 When Should an Adult Be Referred to a Gastroenterologist/
Hepatologist for Management?Recommendation 2.5.1. Persons with persistently elevated ALT or aspartate aminotransferase (AST) levels and/or with hepatic steatosis on imaging and indeterminate risk (FIB-4,1.3- 2.67; LSM, 8-12 kPa; or ELF test, 7.7-9.8) or high risk (FIB-4, >2.67; LSM, >12 kPa; or ELF test, >9.8) based on blood tests and/or imaging (as described in R2.2.1, R2.2.2, and R2.3) should be referred to a gastroenterologist or hepatologist for further assessment, which may include a liver biopsy.
Grade B; Intermediate Strength of Evidence; BEL 2Recommendation 2.5.2. Clinicians should refer persons with clinical evidence of advanced liver disease (ascites, hepatic encephalopathy, esophageal varices, or evidence of hepatic synthetic dysfunction) to a gastroenterologist/hepatologist for further care.
Grade B; Intermediate/High Strength of Evidence; BEL 2Management of NAFLD in Adults
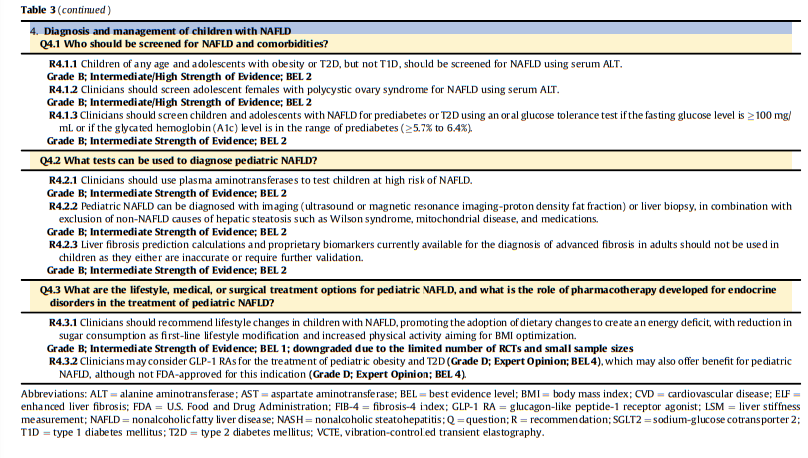
Q3.1 How Should Cardiometabolic Risk and Other Extrahepatic
Complications Be Managed in the Setting of NAFLD?
Recommendation 3.1. Clinicians must manage persons with NAFLD for obesity, MetS, prediabetes, diabetes mellitus, dyslipidemia, hypertension, and CVD based on the current standards of care.
Grade A; High/Intermediate Strength of Evidence; BEL 1fig11 when server ready (Table6 in article)