Many of the instruments that were used in the SPRINT MIND trial are directly applicable to primary care practice for screening for dementia, diagnosing dementia, and monitoring its progression.
These instruments are also applicable to the diagnosis of Mild Cognitive Impairment in primary care practice.
Which instruments a physician chooses to use in his/her practice will depend on circumstances of the practice.
This post is just an extract from the SPRINT protocol focusing on the instruments and methods used in SPRINT MIND. Using this extract, the clinician can choose those instruments and methods that best suit his/her patients.
All that follows is from the SPRINT Protocol [Full Text PDF] (1):
5.4.9 MIND Battery: Dementia Screening
All participants will undergo a dementia screening at baseline and every 2 years thereafter. The tests will include the Montreal Cognitive Assessment (MoCA), Digit Symbol Coding test, and Logical Memory test. A subset of 2800 participants will undergo an additional comprehensive battery of neurocognitive tests conducted at baseline, Month 24, and Month 48. In addition to the neurocognitive tests, a subsample of 640 MIND participants will have a Baseline and Month 48 MRI examination.
Chapter 6 – SPRINT MIND
6.1 SPRINT-MIND Overview
SPRINT-MIND is an integral part of the overall SPRINT study and all SPRINT participants will participate in one or more components of SPRINT-MIND. There are three objectives of SPRINT-MIND. The primary objective is to determine whether a strategy of intensive blood pressure lowering to target systolic blood pressure (SBP) <120 mm Hg versus a standard treatment target of 140 mm Hg will produce a greater reduction in the incidence of all-cause dementia. The second objective is to determine whether global cognitive function measured in key specific domains of cognition will decline less in persons randomized to a SBP goal of <120 mm Hg versus a standard treatment goal of 140 mm Hg in a representative sub-sample of approximately 2800 SPRINT participants. The third objective is to assess whether MRI-derived changes in brain structure differ by treatment assignment in a subset (approximately 640) of the 2800 participants.
6.2 Study Hypotheses and Aims
6.2.1 All-cause Dementia
Primary hypothesis: Over an average of 60 months, the incidence of all-cause dementia will be lower in SPRINT participants assigned to the intensive SBP treatment arm compared to their counterparts assigned to the standard SBP treatment arm. This hypothesis will be tested in all SPRINT participants.
6.2.2 Cognitive Decline
Secondary hypothesis: Over an average of 48 months, the rate of global decline in cognition measured across key domains of cognition will be lower in the intensive SBP treatment arm compared to the standard SBP treatment arm. This hypothesis will be tested in a representative subset of approximately 2800 participants enrolled in SPRINT.
6.2.3 MRI Brain Changes
The Primary brain MRI hypothesis is that over an average of 48 months, the volume small vessel ischemic disease (SVID) will be lower in SPRINT participants assigned to the intensive SBP treatment arm compared to their counterparts assigned to the standard SBP treatment arm. An additional hypothesis is that total brain volume will also be greater (thus less atrophy) in the intensively treated group. The MRI sub-study will be conducted in approximately 640 participants chosen from the 2800 subset of participants selected in 6.2.2.
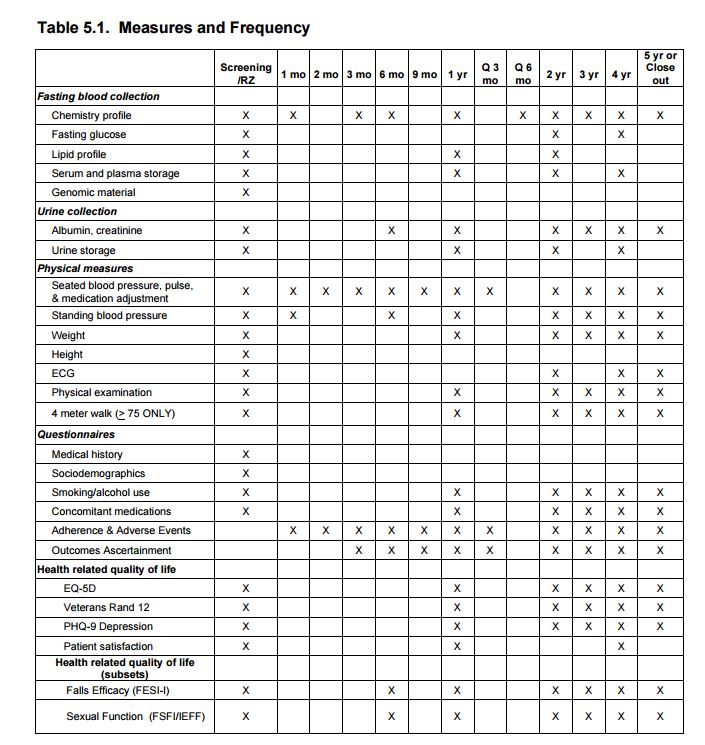
6.3 Study Design 6.3.1 Study Population We will ascertain incident all-cause dementia in all participants enrolled in SPRINT. In addition, approximately 2800 participants will be selected to receive additional cognitive assessments at baseline, 24 months, and 48 months in order to examine changes in global and domain-specific cognition. Participants participating in the MRI substudy will, at baseline, generally be required to reside within 1.5 hours travel distance to a designated study MRI Scanner. The components of the two cognitive batteries selected to assess dementia incidence and decline in cognition are listed in Table 5.1 of Chapter 5
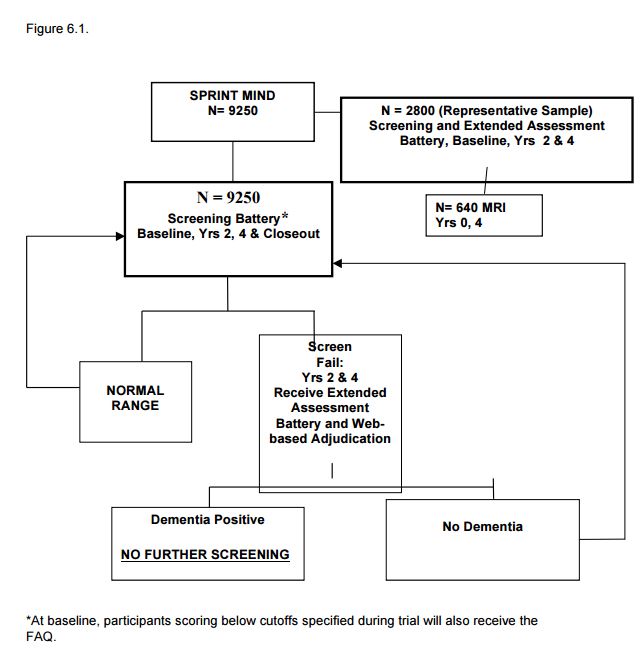
6.4 Procedures for Identifying Incident All-Cause Dementia in SPRINT (see Figure 6.1).
6.4.1 Overview
A 3-step process will be used to ascertain incident cases of all-cause dementia. First, to identify possible cases of dementia a brief Cognition Screening Battery will be administered to all participants. Participants who score below the pre-designated screening cut-point for possible cognitive impairment during follow-up will be administered a more comprehensive and detailed neurocognitive test battery (the Extended Cognitive Assessment Battery) plus the Functional Assessment Questionnaire which assesses impairments in daily living skills as a result of cognitive impairments. Last, all the above available tests and questionnaire data will be submitted to a centralized, web-based system for adjudication by a panel of dementia experts who will assign final study classifications of probable dementia (PD), mild cognitive impairment (MCI) or no impairment (NI).
6.4.2 Cognition Screening Battery
A brief screening battery consisting of 3 well-validated neurocognitive tests will be administered to all participants at study randomization and repeated at years 2, 4 and at closeout (unless the participant has undergone the screening battery in the past year prior to closeout). This battery requires 15 minutes or less to administer.
Tests included in the SPRINT-MIND Cognition Screening Battery were selected because they are sensitive to detecting dementia, easy to administer and brief. They are: 1. The Montreal Cognitive Assessment (MoCA) The MoCA (Nasreddine et al., 2005) is part of the NIH Toolbox and is a reliable and valid brief screening instrument for characterizing global cognitive functioning. It has been used previously to screen for dementia and MCI with sensitivity of >85%. The MoCA has several sub-scales that can be used to characterize more specific cognitive functions. 2. Digit Symbol Coding test (DSC) The DSC ((Wechsler, 1996b; Wechsler D., 1981) is a sub-test of the Wechsler Adult Intelligence Scale-IV. It measures psychomotor speed and working memory. The DSC and its predecessor the Digit Symbol Substitution test have been extensively used and normed. 3. Logical Memory test (LM): The LM test is a sub-test of the Wechsler Memory Scale-IV(Wechsler, 1996a; Wechsler, 1996a). It measures episodic verbal memory and has extensive normative data. Episodic verbal memory is an especially sensitive predictor of early Alzheimer’s dementia and amnestic MCI.
The sensitivity and specificity of the Cognition Screening Battery to detect participants with poorer cognitive function will be evaluated on an ongoing basis during the trial by using available baseline cognition data from SPRINT. We estimate 20-25% of participants will trip the battery (see SPRINT MOP for specific battery cut-points) and receive a brief assessment of the impact of their cognitive function on daily life (the 10 item Functional Assessment Questionnaire). At the years 2 and 4 follow-up, participants who trip the screening battery will also be administered the SPRINT Extended Cognitive Assessment Battery for adjudication of incident dementia. In order to achieve the 20-25% target, various cut-points for the Cognition Screening Battery will be compared and adjustments will be made to maximize study efficiency and economy during the trial.
6.4.3 SPRINT Extended Cognitive Assessment Battery
The Extended Cognitive Assessment Battery will provide a more comprehensive and detailed assessment of specific major cognitive functions (memory, language, visuospatial skills, executive function) that are necessary for classification of dementia and for detecting domain-specific changes. During follow-up years 2 and 4, participants scoring in the impaired range on the Cognition Screening Battery will be administered the Extended Cognitive Assessment Battery at their next scheduled visit (typically a blood pressure assessment and medication distribution visit). This entire battery requires less than 40 minutes including scoring and data entry and less than 30 minutes in persons without significant memory impairment.
The neurocognitive tests comprising the Extended Cognitive Assessment Battery are:
1) The Hopkins Verbal Learning Test (HVLT) (Brandt and Benedict, 2001): A measure of episodic verbal learning and memory, this test is a 12-item list learning and memory task with immediate recall, delayed recall and recognition components.
2) The Trail Making Test: Parts A and B (Reitan R.M., 1958): The Trail Making Test (TMT) is a two-part test measuring speed of processing and executive function. The times to complete Part A and Part B are the primary measures of interest.
3) Digit Span test (Wechsler D., 1981): The Digit Span test (DST), a subtest of the Wechsler Adult Intelligence Scale-IV, requires the participant to recite gradually increasing series of digits forward and backward. The DST measures concentration and working memory.
4) The Boston Naming Test (Kaplan E et al., 1983) The Boston Naming Test (BNT) is used to assess language function. The participant is asked to name familiar objects from simple drawings. The number of correctly identified objects is the variable of interest. We will use a validated short form that includes 15 items.
5) The Modified Rey-Osterrieth Complex Figure (mRey-O). (Saxon, 2003) The mRey-O measures of visuospatial and visuomotor function and non-verbal memory by having participants copy and reproduce from memory a multicomponent figure. For ease of use and scoring reliability, the mRey-O figure will be faxed to the CC and scored centrally.
6) Category Fluency-Animals. The animal fluency task requires the participant to spontaneously name as many animals as possible in 60 seconds. It provides an assessment of semantic fluency.
6.4.4 Additional measures
Functional Assessment Questionnaire (FAQ). Since impairment of daily functioning is required for a classification of dementia, we also will administer, either locally (by certified SPRINT clinic staff) or centrally (by certified SPRINT staff from the coordinating center), the FAQ, a 10-item, validated questionnaire assessing functional status (Pfeffer and others, 1982), to a person previously designated by the participant who is familiar with his/her current abilities. Administration of the FAQ will be limited to participants whose Cognition Screening Battery indicates possible impairment. Items assess functions like managing money and remembering names of familiar persons.
6.4.5 Alternative cognitive assessment.
If participants cannot come to the clinic for their follow-up exams or if they reside in nursing homes, study personnel will complete either a home or nursing home visit. Technicians conducting the home visit must be MIND certified. The Screening Battery will be administered and if the participant scores below the cut-point, the Extended Battery will also be administered. Telephone assessment of general cognitive function is now standard practice in many large trials assessing for dementia outcomes. For SPRINT participants unable to receive a face-to-face cognitive assessment by certified SPRINT staff at their local clinic, a telephone assessment of cognition status to assess for incident dementia will be performed either locally or centrally by SPRINT certified staff. The components of the phone interview are:
Modified Telephone Interview for Cognitive Status (TICS-M), a validated instrument requiring <10 minutes (Welsh, 1993) Oral Category Fluency-AnimalsOral Trail Making Test (Ricker et al., 1996)FAQ to a contact [The Functional Assessment Questionnaire – above]
For participants unable to be interviewed in-person or by phone, a previously identified contact will be administered:
The Dementia Questionnaire (DQ). The DQ (Ellis , 1998;Kawaset al, 1994) is a semi-structured interview designed for a knowledgeable proxy to provide information regarding the participant’s cognitive and behavioral functioning and other health information needed to make a diagnosis of dementia and MCI and to identify causes of cognitive impairment. Again, it will only be administered in the absence of an in-person or phone assessment and may be performed either by local or central staff who are SPRINT certified.
6.5 Adjudication of Dementia, MCI or No Impairment
A primary goal of SPRINT MIND will be to determine the incidence of all-cause dementia in SPRINT and its relation to the treatment assignment. Final classification (Dementia, MCI or No Impairment) will be made by a panel of experts consisting of neurologists, geriatricians, psychiatrists and neuropsychologists with recognized expertise in dementia blinded to study assignment and blood pressure data. Data used in the adjudication will include all available cognitive test data (SPRINT Cognition Screening Battery, SPRINT Extended Cognitive Battery), functional status assessments (FAQ or DQ) and additional data including demographic information and medical historyEach suspected case identified by our scoring criteria (see 6.4) will be randomly assigned to two members of the Adjudication Committee for review. Adjudicators will independently review all the available data via a web-based system before recording their classification-Dementia, MCI or No Impairment. Each adjudicator will be masked to the other’s classification and to the participant’s treatment assignment. If the two adjudicators’ classifications agree, then the classification will become final. Disagreements will be resolved at periodic face-to-face meetings or by phone conferences between adjudicators and additional members of the Adjudication Committee until consensus is achieved. These procedures have been successfully used by our team in other large clinical trials including the Gingko Evaluation of Memory Study (GEMS) (DeKosky et al, 2008) and the Women’s Health Initiative Memory Study (WHIMS) (Shumaker et al, 2004). Participants classified as having dementia will no longer be assessed for cognitive function. Those not classified as having dementia will continue to receive regularly scheduled cognitive assessments with the screening and extended cognitive batteries when indicated.
6.5.1 Diagnostic Criteria for Dementia
Criteria used for identifying dementia will be those described in the Diagnostic and Statistical Manual of the American Psychiatric Association-Fourth Edition (DSM-IV). These are:
• Significant decline in memory and at least one additional cognitive domain; and
• Significant functional impairment due to cognitive problems; and
• Cognitive deficits are not due to obvious reversible causes such as acute illness, metabolic disturbances, infections, mood disorders or substanceinduced conditions; and cognitive deficits do not occur exclusively during the course of delirium. No attempt to classify dementia subtype will be made.
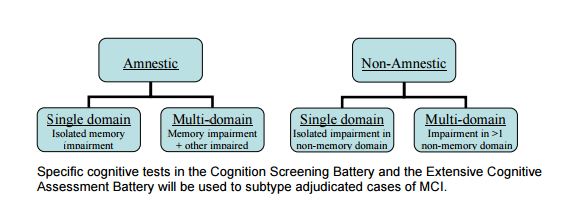
6.5.2 Diagnostic Criteria for MCI
While not a primary or secondary outcome, MCI syndrome is important because of its relevance to dementia. MCI represents a transitional state between no cognitive impairment and dementia and specific subtypes of MCI are highly predictive of subsequent dementia. Thus, identifying MCI will provide valuable information about predementia cognitive impairment related to the SPRINT intervention. Criteria to be used for identifying mild cognitive impairment syndrome are those described by Winblad et al., which are:
• Observation by participant or proxy of cognitive decline; and
• Deficit in performance in one or more cognitive domains; and
• Absence of significant functional impairment attributable to cognition; and
• No diagnosed dementia MCI will be further sub-classified into 4 categories using criteria adapted from Winblad, et Al. (Winblad et al, 2004) as follows:
6.6 Baseline classification of cognitive status:
Rare cases of dementia, where the participant or their personal physician are unaware of the diagnosis, may be identified during baseline cognitive testing. In participants scoring below the cut-point on the Screening Battery, we will administer the FAQ to a contact in order to determine the presence of impaired daily function related to cognition (see 6.4.2).
6.7 Definition of Cognitive Change Over Time Outcome (Extended Cognitive Assessment Battery Sub Sample).
Each test score from the Cognition Screening Battery and the Extended Cognitive Assessment Battery will be used to measure decline in cognitive function. The primary outcomes will be composite scores for two domains: 1) Memory, consisting of the Hopkins Verbal Learning Test, Logical Memory and the Modified Rey Osterrieth Figure, and 2) Processing Speed, consisting of Trails Making Tests and Digit Symbol Coding Test. Prior to analysis of this outcome, we will review the science related to summary scores for cognitive function and may make modifications which will be specified prior to initiation of the analysis.
6.8 Quality Control and Training At each clinical site, at least one person will be identified to serve as the trained and certified cognitive technician. Technicians will be trained during a central, intensive training session held in conjunction with the overall SPRINT training. Training will include review of the MIND protocol and procedures for administration of the test batteries, demonstrations of each component of the SPRINT MIND test batteries, and opportunities to practice with feedback from trainers. When a level of competence is attained, technicians will receive certification and approval to administer the test batteries to SPRINT participants. During the course of the study as additional staff is needed, certified technicians will train new technicians and submit materials to the MIND Coordinating Center for review. Technicians will be recertified throughout the course of the trial by: 1) review of video or audio taped administrations; 2) observing web-based administrations and responding to specific questions. Technicians will be encouraged to communicate questions or problems to the SPRINT MIND Coordinating Center.
Chapter 7 – Health-Related Quality of Life and Economic Analyses
7.1. Introduction
In addition to the cardiovascular, renal and cognitive outcomes, SPRINT is well poised to examine differences in health-related quality of life (HRQL) as a result of its blood pressure interventions. Differences in HRQL may affect adherence, and thus the effectiveness of the two interventions. It is also reasonable to anticipate that in some cases, the intensive arm may result in diminished HRQL relative to the standard arm due to a number of factors:
• side effects of specific medications or increased numbers and/or doses of medications required to achieve the <120 mm Hg goal,
• increased occurrence of hypotensive symptoms, which may not only result in higher rates of falls and fractures, but also an increased fear of falling which may limit the participant’s perceived ability to engage in activities of daily living, and/or
• reduced perfusion pressures and medication side effects which may contribute to erectile dysfunction in men, and possible sexual dysfunctions in women.
On the other hand, the intensive arm may result in improved general HRQL versus the standard arm due to reduced number of medical events and more favorable physical and cognitive function. The effects of the two interventions upon HRQL are further nuanced by the possibility that some participants in either treatment arm may adjust to decrements in health status by changing their internal perception of favorable HRQL, known as “response shift”. There may also be potential health cost tradeoffs of the intensive versus standard treatment. While the intensive arm is anticipated to result in higher short-term costs due to more frequent office visits and greater medication use, this arm may also result in lower long-term costs from event-related hospitalizations and other medical costs if the treatment approach is efficacious in reducing these medical events. Assuming the primary outcomes are as hypothesized, examining the HRQL and cost-effectiveness of the intensive and standard treatment arms will be important determinants of the potential adoption of the intensive BP control in clinical practice, and will be informative in identifying subgroups of patients for whom intensive or standard BP control is most appropriate.
7.2. Hypotheses
7.2.1 HRQL Hypotheses
The hypotheses generated for the HRQL measures are:
• Overall HRQL (Entire sample, Veterans RAND-12) Intensive control of blood pressure compared to standard control will result in worse HRQL at the 1-year assessment, but better HRQL at the 5-year assessment. The effect will be greater in those with lower baseline HRQL and greater number of comorbid conditions at baseline.
• Falls Self-efficacy (Subsample, Falls Self Efficacy Scale) Intensive control of blood pressure compared to standard control will result in less favorable fall-related self-efficacy at the 1-year assessment. The effect will be the greater in older participants, those with lower baseline HRQL, and those with a greater number of baseline comorbid conditions. By Year 5, intensive control of blood pressure will result in more favorable fall-related self-efficacy compared to standard control.
• Sexual function (Subsample, Modified Female Sexual Function Assessment /International Index of Erectile Function) Intensive control of blood pressure compared to standard control will decrease sexual function among men and women participants at one year. By year 5, the intensive treatment participants will report more favorable sexual function compared to participants in the standard treatment.
7.2.2 Cost-Effectiveness Hypotheses [See p 46]
7.3. Health-Related Quality of Life Measures
7.3.1 Rationale for Selection
The SPRINT HRQL instruments were selected based upon the following criteria: (1) inclusion of the major dimensions shown in the literature to be affected by hypertension and its treatment; (2) brevity; (3) responsiveness to treatment-related changes, and (4) appropriateness for the age range, racial/ethnic diversity, and anticipated medical conditions of the participants in SPRINT. To reduce participant burden, some HRQL instruments will be administered to the entire SPRINT sample, while others will be administered only in a subsample of participants. All HRQL instruments will be self-administered unless participants require assistance due to sensory, motor, or cognitive deficits in which case the instruments will be administered by clinic staff or family/friends accompanying the participant to the clinic visit. For Spanish-speaking participants, Spanish versions of all HRQL instruments will be administered to participants at all assessment points who indicate at baseline that they do not have sufficient written English fluency to complete the instruments in English.
7.3.2 Health-Related Quality of Life (HRQL) Measures
Veterans RAND 12-item (VR-12) questionnaire. The VR-12 is a shorter version of the VR-36 (which is derived from the SF-36). Changes of the VR-12 relative to the SF-12 have lowered the floor and ceiling, improved the distributional properties, increased reliability, and improved discriminant validity of the physical and mental health summary scores. Validated conversion formulas allow for direct comparisons to prior studies using the SF-36 or SF-12. The VR-12 will be administered to all SPRINT participants at baseline and at annual visits thereafter.
Fall Self-Efficacy Scale International (FES-I) The FES-I, shortened version, consists of seven items which the respondent answers on a 1-4 scale, indicating level of concern for falling. The activities are getting dressed or undressed, taking a bath or shower, getting in or out of a chair, going up or down stairs, reaching for something above your head or on the ground, walking up or down a slope, and getting out to a social event. An evaluation of the Short FES-I found good internal and 4-week test-retest reliability. The correlation between the Short FES-I and the FES-I was 0.97. The Short FES-I will be
administered among a subsample of SPRINT participants.International Index of Erectile Function (IIEF) The IIEF-5 is the 5-item short form of the original 15-item IIEF, and was developed specifically for use in clinical settings to supplement physical examination and patient history. IIEF-5 scores can be classified into the following categories; severe erectile dysfunction (ED), moderate ED, mild to moderate ED, mild or no ED. Scores less than 21 have 98% sensitivity and 88% specificity for the presence of ED. The IIEF-5 will be administered in a male subsample of SPRINT participants.
Female Sexual Function Assessment (FSFI) The FSFI is a 19-item survey that assesses female sexual function over the past four weeks in 6 domains (desire, arousal, lubrication, orgasm, satisfaction, and pain). Utilizing recently proposed modifications to the FSFI, participants not sexually active over the past four weeks would complete only 4 items, substantially reducing respondent burden. The FSFI has high internal
consistency (Cronbach alpha > 0.8). This assessment will be administered in a female subsample of SPRINT participants.
Patient Satisfaction (Bharmal and others, 2009) A modified Treatment Satisfaction Questionnaire for Medication (TSQM) General Satisfaction subscale will be administered at baseline (based on current blood pressure medications being taken, if any) and at 1 and 4 years. This corresponds with the administration of the Morisky Adherence scale, which will allow for analyses of the relationship between satisfaction and adherence at these time points.Patient Health Questionnaire-9 (PHQ-9) The PHQ-9 is a self-report measure of depression that has been recommended by the AHA Advisory Panel on Depression and
Coronary Heart Disease, has a low response burden (9 items; 2-3 minutes to complete), excellent reliability, and good sensitivity and specificity with depression diagnoses. This assessment will be done at baseline and annually on all participants.7.3.3 Health State Utility Measures
EQ-5D is a self-administered 5-item instrument including mobility, self-care, usual activities, pain/discomfort and depression. There are three responses to each question (no, moderate, or severe limitations). This commonly used measure of health utilities will be used to convert quality of life and health status into quality adjusted life-years (QALYs) for cost-effectiveness analysis. The EQ-5D will be administered to all participants at baseline and annually thereafter.
Resources:
(1) Systolic Blood Pressure Intervention Trial (SPRINT)
Protocol Version 4.0 November 1, 2012 [Full Text PDF]
Links to The SPRINT Trial and to NEJM Resources and to WWW.SPRINTTRIAL.ORG Resources, Nov 11, 2015. tomwademd.net