What follows are excerpts from the European Society of Cardiology Resource (1) 2017, ESC working group position paper with non-obstructive coronary arteries:
[DeWood’s] pioneering studies demonstrated that, in patients presenting with ST elevation myocardial infarction (STEMI), almost 90% had an occluded coronary artery provided that angiography was undertaken within 4 h of chest pain onset.5 In contrast, in AMI patients who did not present with ST elevation (non-ST elevation myocardial infarction or NSTEMI), only 26% had an occluded coronary artery when angiography was performed within 24 h of symptom onset.6 In both of these landmark studies,5,6 .90% of the acute MI patients had angiographic evidence of obstructive coronary artery disease (CAD), underscoring the importance of the atherosclerotic process in the pathogenesis of AMI.
Although DeWood’s studies underscore the importance of obstructive CAD in AMI, it is fascinating that 10% had no significant CAD on coronary angiography. This is confirmed in several large AMI registries7 – 9 where 1–13% of AMI’s occurred in the absence of obstructive CAD thereby eliciting an important set of questions—what is the mechanism of the myocardial damage in these patients? Do these patients differ from those with obstructive CAD? Should they be managed with the same clinical strategies? Do patients with non-obstructive atherosclerosis differ in pathophysiology and outcomes from those with angiographically normal coronary arteries? These and other issues have prompted clinical researchers to coin the term myocardial infarction with nonobstructive coronary arteries (MINOCA). This position paper is the first authoritative international expert opinion regarding this intriguing condition. We seek to define MINOCA, describe its associated clinical features and mechanisms, detail an assessment pathway for its evaluation, as well as stimulate research into its mechanisms and treatment.10
Rationale for diagnosis
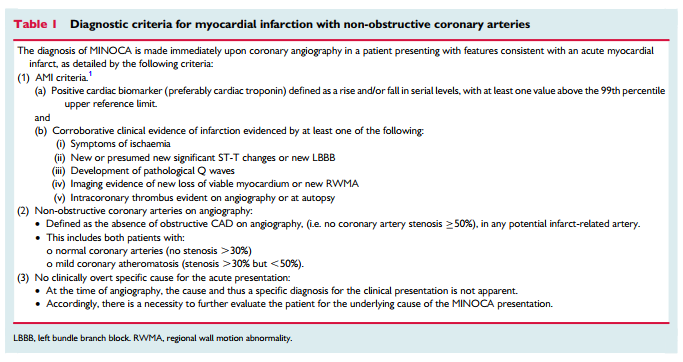
As the name implies, the formulation of the MINOCA diagnosis requires both clinical documentation of an AMI and demonstration of non-obstructive coronary arteries (Table 1). Accordingly, the diagnosis is made following coronary angiography in the evaluation of a clinical presentation consistent with AMI.
One of the required components of the diagnosis of acute myocardial infarction is an elevated troponin.
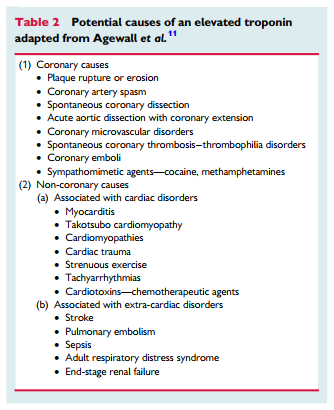
There are three central concepts in interpreting troponins in the context of a coronary angiogram showing non-obstructive coronary arteries. First, it should be appreciated that cardiac troponins are ‘organ specific’ and not ‘disease specific’. An elevated cardiac troponin is not necessarily indicative of an AMI but reflects myocardial injury or necrosis. One example of a disease process causing myocardial injury and troponin elevation without an AMI is pulmonary embolism. Thus, there must be corroborative clinical evidence in addition to elevated cardiac troponins to establish the diagnosis of AMI, including MINOCA. However, there is no imaging technology, including cardiac magnetic resonance (CMR) imaging, which can de- finitively exclude an ischaemic cause of troponin elevation (see ‘MINOCA with normal cardiac MR imaging’). Only a pathologic examination is definitive. Second, rarely the troponin assays may provide spurious results due to analytical issues such as heterophilic antibodies.11 Finally, there are several differential diagnoses for MINOCA, which may arise from both coronary and non-coronary mechanisms as listed in Table 2 and summarized later in this paper. Certainly, the presence of coronary atherosclerotic obstructions, does not exclude other non-cardiac causes of troponin rise.
Coronary artery spasm may be the underlying cause of MI in patients with or without atherosclerosis. Therefore, we believe the best approach is to define MINOCA on the basis of absence of a potentially obstructive stenosis on coronary angiography rather than on the presence or absence of any coronary atherosclerosis.
The authors make the above statement because normal coronary angiography does not rule out coronary atherosclerosis.
The more sensitive techniques for the detection of coronary artherosclerosis, intracoronary ultrasound and intracoronary optical coherence tomography are not routinely used during cardiac catheterization. And the authors note that we do not yet have information on whether routine use of these two imaging techniques will add beneficial information to coronary angiography.
Intracoronary imaging at the time of cardiac catheterization with intravascular ultrasound (IVUS) or optical coherence tomography
(OCT) may be useful to identify atherosclerotic plaque disruption and plaque erosion as well as coronary dissection or thrombosis, which may not have been appreciated during angiographyClinical assessment
As stated above, MINOCA is a working diagnosis and should lead the treating physician to investigate underlying causes, analogous to heart failure. This section outlines suggestions for diagnostic testing in order to identify or exclude potential aetiologies discussed in ‘Differential diagnosis’. Left ventriculography or echocardiography should be performed in the acute setting to assess wall motion. This will aid the clinician in determining whether takotsubo cardiomyopathy is a diagnostic consideration. Cardiac magnetic resonance imaging is the key diagnostic tool to be employed in MINOCA patients. Late gadolinium enhancement (LGE), when present, permits localization of the area of myocardial damage and provides insight into mechanisms. For example, an area of LGE in the subendocardium suggests an ischaemic cause of injury, although it does not identify the particular cause of ischaemia (plaque disruption, vasospasm, thromboembolism, or dissection), while a sub-epicardial localization speaks in favour of cardiomyopathy. In other patients, a non-ischaemic appearance of LGE may suggest a diagnosis of myocarditis or an infiltrative disorder. Imaging for myocardial oedema and contractile function may also help localize the area of injury, though with less mechanistic insight.
We recommend that clinicians consider pulmonary embolism as a possible cause of myocardial damage and exclude this diagnosis with D-dimer testing (usually however also elevated in the setting of AMI) and/or computed tomography (CT) pulmonary angiography as appropriate.17
. . . Furthermore, it is important to consider type-2 AMI1 causes, in which a condition other than coronary plaque instability contributes to an imbalance between myocardial oxygen supply and demand and cause myocardial necrosis, such as tachyarrhythmia, haemorrhage, sepsis, and hypertensive crisis, as potential causes of MINOCA.
However, no pulmonary embolism was found among 100 consecutive patients with MINOCA who underwent CT pulmonary angiography in one series.18 Furthermore, it is important to consider type-2 AMI1 causes, in which a condition other than coronary plaque instability contributes to an imbalance between myocardial oxygen supply and demand and cause myocardial necrosis, such as tachyarrhythmia, haemorrhage, sepsis, and hypertensive crisis, as potential causes of MINOCA
After considering clinically apparent diagnoses, the most common causes of MINOCA that the treating clinician must consider are plaque rupture or erosion, coronary artery spasm, thromboembolism, coronary dissection, takotsubo cardiomyopathy, unrecognized myocarditis, and other forms of type-2 myocardial infarction.
Aetiologic differential diagnosis
Plaque disruption
Atherosclerotic plaque disruption is a frequent cause of MINOCA. Plaque disruption is comprised within type-1 AMI in the Universal Definition of Myocardial Infarction, even when no thrombus can be found.1 Within the Universal definition document, MINOCA comprises 5–20% of all type-1 AMI cases. The term disruption encompasses imaging and pathologic findings of plaque rupture, ulceration, or erosion. Intraplaque haemorrhage may also play a role. Two independent studies using intravascular ultrasound identified plaque rupture or ulceration in 40% of patients with MINOCA.19,20.
Coronary artery spasm
Coronary artery spasm may potentially contribute to the pathogenesis of AMI in patients with obstructive CAD and particularly warrants close consideration in those with MINOCA. It reflects a vascular smooth muscle hyper-reactivity to endogenous vasospastic substances (as in vasospastic angina) but may also occur in the context of exogenous vasospastic agents (e.g. cocaine or metamphetamines).30 Provocative spasm testing has demonstrated inducible spasm in 27% of patients with MINOCA suggesting that it is a common and an important pathogenetic mechanism in MINOCA.31 Considering that nitrates and especially calcium channel blockers are effective therapies for coronary artery spasm, with the latter shown to prevent cardiac events in vasospastic angina, this diagnosis and treatment needs to be carefully contemplated.
Myocardial infarction with non-obstructive coronary arteries may be the de novo presentation for patients with vasospastic angina, or an interim event in those with the chronic established form of the disorder. Clinical features of vasospastic angina that may allude to the diagnosis in patients with MINOCA include recurrent episodes of rest angina that promptly respond to short-acting nitrates, especially if associated with transient ischaemic ECG changes and demonstrating a circadian pattern (typically as nocturnal angina).
Thus, a diagnosis of vasospastic angina can be made if spontaneous episodes of rest angina are associated with ST-segment changes that respond promptly to short-acting nitrates. However, spontaneous episodes are infrequently documented therefore requiring provocative spasm testing to be undertaken if the diagnosis is to be pursued.Coronary thromboembolism
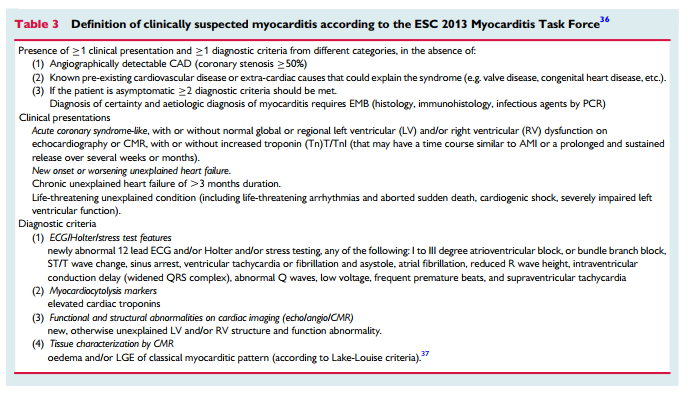
Thrombosis may be a contributory mechanism to AMI in the setting of plaque disruption or coronary artery spasm, or may be the cause of MI in the absence of these factors. Coronary thrombosis may
arise from hereditary or acquired thrombotic disorders and coronary emboli may occur from coronary or systemic arterial thrombi. Hereditary thrombophilia disorders include Factor V Leiden thrombophilia, Protein S and C deficiencies. Thrombophilia screening studies in patients with MINOCA have reported a 14% prevalence of these inherited disorders.31 Acquired thrombophilia disorders should also be considered such as the antiphospholipid syndrome and myeloproliferative disorders, although these have not been systematically investigated in MINOCA.
Coronary dissection
Spontaneous coronary dissection typically causes an AMI via luminal obstruction, although this may not always be apparent on coronary angiography, prompting a diagnosis of MINOCA.38 Intramural haematoma of the coronary arteries without intimal tear presents similarly.39 Intracoronary imaging is pivotal in making this diagnosis.40 The condition is more common among women. The reasons for the occurrence of coronary dissection are still unclear but fibromuscular dysplasia is present in other vascular beds in the majority of cases when screening is performed.41
Takotsubo cardiomyopathy
Takotsubo cardiomyopathy44 often presents as an acute coronary syndrome with ST segment changes.45,46 The transient nature of left ventricular dysfunction has puzzled physicians worldwide.47 Clinical presentation is characterized by acute, reversible heart failure associated with myocardial stunning, in the absence of occlusive CAD.48 The prognosis is generally good although several studies have demonstrated significant complications in the acute phase49 – 52 and more studies with long-term follow-up are required
Myocarditis
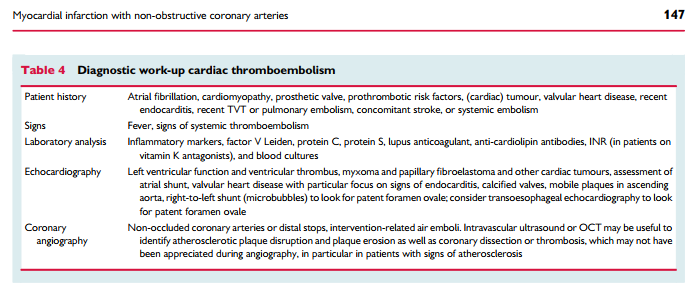
Since clinical presentation is polymorphic, the 2013 ESC Task Force has introduced rigorous criteria for clinically suspected myocarditis36 that are outlined in Table 3. Certain diagnosis of myocarditis and of its specific aetiopathogenetic forms can only be achieved by endomyocardial biopsy (EMB).36
The prevalence of myocarditis among patients with a clinical diagnosis of MINOCA varies based on the populations studied, with a prevalence of 33% in a recent meta-analysis.55 The most common cause of biopsy-proven myocarditis is viral infection, confirmed with polymerase chain reaction (PCR) assay of the pathogen DNA/RNA on EMB. Other causes of myocarditis are immunemediated diseases, endocrine diseases, drugs, and toxins.36,56 – 58 Autoimmune myocarditis may occur with exclusive cardiac involvement or in the context of systemic autoimmune disorders, e.g. systemic
lupus erythematosus and is infection-negative by PCR on EMB.36,59,60The initial investigation of suspected myocarditis should include CMR imaging. Although this non-invasive investigation compares favourably with the gold-standard technique of EMB,36,56,61,62 only EMB provides the opportunity of identifying the underlying cause for the myocarditis. Lurz et al.63 reported that CMR imaging detected 79% of EMB-confirmed myocarditis. Also, in the new ESC guidelines on Pericardial disease CMR is recommended for the confirmation of myocardial involvement (myocarditis) as a Class I recommendation.64
The importance of diagnosing myocarditis in patients with MINOCA relates to its prognosis and treatment. Although myocarditis resolves over a 2 –4 weeks period in 50% of patients, 12–25% may acutely deteriorate and either sucumb to fulminant heart failure or progress onto end-stage dilated cardiomyopathy requiring heart transplantation.36
Other forms of type-2 acute myocardial infarction
Type 2 AMI is defined as myocardial cell necrosis due to supply –demand mismatch, characterized by significant increase and/or decrease in troponins with at least one value above the 99th percentile of a normal reference population in the absence of evidence for coronary plaque rupture in addition to at least one of the other criteria for AMI.1 Major determinants of myocardial oxygen demand include systolic wall tension, contractility, and heart rate, while myocardial oxygen supply is conveyed by coronary blood flow and oxygen content. Conditions underlying type-2 AMI include anaemia, tachy- brady-arrhythmia, respiratory failure, hypotension, shock, severe hypertension with or without left ventricular hypertrophy, severe aortic valve disease, heart failure, cardiomyopathy, and injurious effects of toxins (e.g. sepsis) and pharmacological agents (e.g. catecholamines).65
Myocardial infarction with non-obstructive coronary arteries of uncertain aetiology
When CMR is normal and diagnostic evaluation as recommended herein does not reveal the mechanism of AMI, there is a diagnostic and therapeutic dilemma for clinicians. Unfortunately, there are no systematic investigations addressing this issue. From first principles, vasospastic angina, coronary plaque disruption, or thromboembolism may all potentially cause MINOCA with normal CMR imaging. In a series of patients with MINOCA who underwent both CMR and IVUS imaging, a subset of those with plaque disruption had a normal CMR (25%).19 If intracoronary imaging had not been performed during cardiac catheterization, this diagnosis would have been missed. Furthermore, MINOCA studies undertaking provocative spasm testing or assessing microvascular dysfunction have not routinely performed before CMR. However, epicardial coronary artery spasm may produce transient transmural myocardial ischaemia that is associated with a small troponin rise.34 An alternative consideration is that the troponin rise is not indicative of AMI and is instead due to other causes such as pulmonary embolism or myocarditis. These alternate causes should be reconsidered when CMR is normal.
Resources:
(1) ESC working group position paper on myocardial infarction with non-obstructive coronary arteries [PubMed Citation] [Full Text HTML] [Full Text PDF]. Eur Heart J. 2017 Jan 14;38(3):143-153. doi: 10.1093/eurheartj/ehw149.
(2) Troponin elevation in coronary vs. non-coronary disease [PubMed PDF] [Full Text HTML]. Eur Heart J 2011;32:404–411.