Today, I link to, and excerpt from The Impact of Cancer Screening on All-Cause Mortality: What Is the Best We Can Expect? [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Dtsch Arztebl Int. 2018 Jul 23;115(29-30):481-486. doi: 10.3238/arztebl.2018.0481.
There are 84 similar articles in PubMed.
The above article has been cited by 15 articles in PubMed.
All that follows is from the above article.
Abstract
Background: It is a matter of debate whether, and if so, to what extent, cancer screening programs reduce all-cause mortality. Against this backdrop, we analyzed potential effects of several cancer screening approaches on all-cause mortality in two representative Western European populations.
Methods: We used mortality data from the UK (England &Wales) and Germany from 2015 and published figures from screening studies on relative reduction in mortality for screened cancers to calculate the expected decline in all-cause mortality in these countries. We determined the required sample size for demonstrating a 3% reduction in all-cause mortality with a narrow (95%) confidence interval in a hypothetical screening trial.
Results: A relative 20% reduction in breast cancer mortality can be accompanied by a maximum 1.7-1.8% reduction in all-cause mortality in England & Wales and Germany, respectively. Expected declines are smaller for sigmoidoscopy screening (1.0-1.2%), prostate-specific antigen (PSA) screening (0.4-0.6%), and skin cancer screening (0.2%). To obtain a 95% confidence interval of +/-1% for demonstrating a 3% decline in all-cause mortality, a study size of 596 200 persons is required.
Conclusion: Because the proportion of cancer deaths in all deaths in Western Europe is relatively low, cancer screening procedures can reduce all-cause mortality by only 1-3%. However, this reduction is relevant to public health.
Key messages
There are three death categories for the calculation of mortality rates in screening studies: a) cancer-related death in a person suffering from the cancer of interest, b) death in a person suffering from the cancer of interest which is not attributed to the cancer of interest, and c) death of any cause in persons not suffering from the cancer of interest. Many screening studies report cancer-specific mortality rates (disease-specific mortality rates), which only takes category a) into account. Some screening studies also report all-cause mortality rates, taking into account deaths of all categories a)–c).
Imagine that in one study arm of a screening study a total of 10 000 person-years were spent on follow-up. 50 participants died of the cancer of interest. Another 30 participants, who also fell ill with the cancer of interest, died of another cause. Another 20 participants who did not fall ill with the cancer of interest during their lifetime died from another cause. The cancer-specific mortality rate is therefore calculated as 50 per 10 000 person-years, while the all-cause mortality rate is calculated as 100 per 10 000 person years.
Opponents of the use of the all-cause mortality rate state that even common cancers account for only a small proportion of the total number of deaths and therefore screening trials would require sample sizes too large to be feasible. However, the expected decline in all-cause mortality after the introduction of cancer screening in populations like Western Europe have not been estimated (2).
We could not find any publication that quantifies the expected decline of the all-cause mortality rate if an efficacious screening for a specific cancer is introduced. Knowledge about the expected decline of the all-cause mortality rate helps to interpret results from statistically underpowered screening studies. For example, the European Randomized Study of Screening for Prostate Cancer (ERSPC) revealed that the prostate cancer-specific mortality rates among men aged 55–69 years over a period of 11 years decreases by 21% if prostate-specific antigen (PSA) screening is conducted every four years (5). This study showed that the all-cause mortality rates were very similar (screening: 18.2 per 1000 person-years; no screening: 18.5 per 1000 person-years; mortality rate ratio 0.99, 95% CI: [0.97; 1.01]). Based on these results, Schröder et al. stated, “In our study, there was no effect on all-cause mortality.” (5) Are Schröder et al. right?
The aim of this paper is to present the potential effect of cancer screening on all-cause mortality in Western Europe. We chose two representative countries for which recent mortality data were available. We hereby assess the role of the magnitude of the cancer mortality rate without screening and the relative reduction in the cancer mortality rate due to screening.
Material and methods
We estimated age-standardized mortality rates for all-cause mortality and for screening-detectable cancers by use of the European Standard Population (7). We compared the sex- and age-specific mortality rates of these cancers graphically. We used estimates of the relative rate reduction (RRR) of cancer mortality for screenings that have been studied by large randomized controlled trials including PSA screening (age 55–69 years) (5), mammography screening (age 50–69 years) (8), and flexible sigmoidoscopy screening (age 55–64 years) (9).
We used a RRR of 21% for PSA screening (5), 20% for mammography screening (8), 27% for flexible sigmoidoscopy (9), and 50% for skin melanoma (10) respectively. For skin cancer, we only focused on skin melanoma deaths as non-melanoma skin cancer mortality rates are very low. We used the RRR to estimate cancer-specific mortality rates, assuming a scenario where screening is applied to 100% of the eligible population, and thereafter calculated the expected all-cause mortality rate, accounting for the expected reduction in cancer-specific mortality due to screening. This calculation assumes that the all-cause mortality rate is directly influenced only by the change in the cancer-specific mortality rate. Indirect effects such as, for example, suicide after a diagnosis of cancer may decrease the effect.
We thereafter calculated the mortality rate ratio (rate in presence of screening/rate without screening) for all-cause mortality.
In addition, we investigated the hypothetical effect of screening in ischemic heart disease (ICD-10: I20–I25), whose disease-specific mortality rate (and therefore its proportion of all-cause mortality) in the 45–69 year-old age group is considerably higher than that of cancer. In a sensitivity analysis, we assumed that the screening effect would also be noticeable in the 5-year age group above the approved screening age (e.g. in mammography screening the group of 70–74 year-olds) if we used the same RRR for this group.
In order to analyze the dependence between the relative rate reduction of a screening program for a specific cancer and the cancer mortality rate of this cancer without screening, which would exist for a reduction in all-cause mortality by 1%, 2%, or 3%, respectively, we derived a mathematical formula.
To calculate the required sample size for estimating a relative risk of 0.98 or 0.97 of all-cause mortality in a hypothetical screening trial with a narrow two-sided 95% confidence interval of +/–0.01, we used the confidence interval method by Katz et al. (11). We assumed equal group sizes for screened and unscreened participants of a hypothetical randomized controlled screening trial.
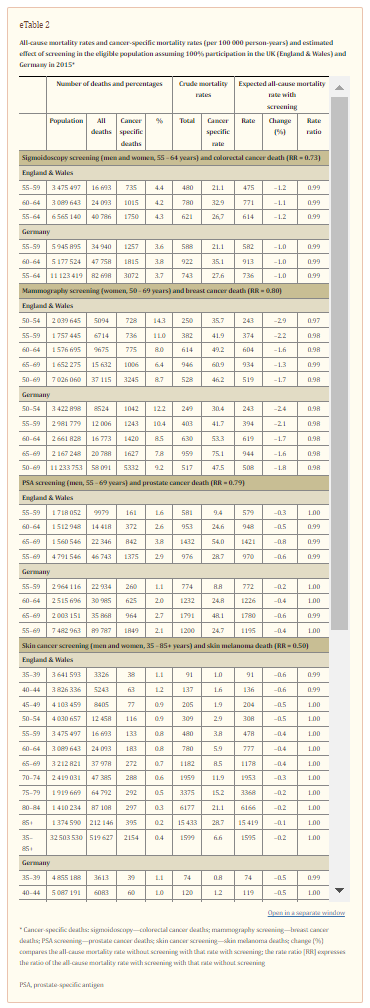
A cancer screening procedure among people aged 50–74 years with a relative rate reduction (RRR) in cancer-specific mortality of e.g. 20% that would also be associated with a reduction in all-cause mortality by 1%, 2%, or 3% among men would require a mortality rate of that cancer without screening of 55, 110, or 165 per 100 000 person-years, respectively, in the UK (England & Wales) and Germany (women: 35, 69, or 104 per 100 000 person-years, respectively) (figure).
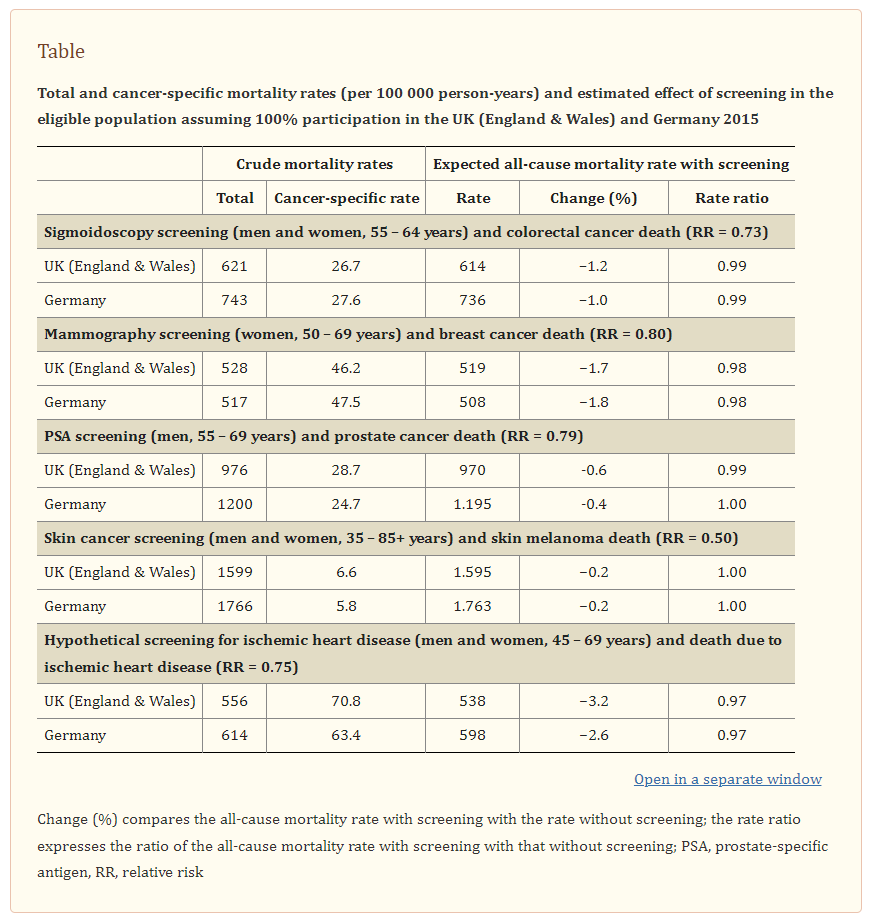
The Table shows the expected decline in all-cause mortality among screening-eligible populations with 100% participation in a cancer-specific screening programme for the UK (England & Wales) and Germany for colorectal cancer, breast cancer, prostate cancer, and skin melanoma. The proportion of cancer-specific mortality among all-cause deaths is 8.7% and 9.2% for women aged 50–69 years in the UK (England & Wales) and Germany, respectively.
Despite these high proportions, a RRR in breast cancer mortality within that age group would produce a relative decline in the all-cause mortality rate of only 1.7% and 1.8% in the UK (England & Wales) and Germany, respectively. Relative declines in the all-cause mortality rate would be smaller for sigmoidoscopy screening (1.0–1.2%), PSA screening (0.4–0.6%), and skin cancer screening (0.2%).
[BUT] A hypothetical screening for ischemic heart disease among people aged 45–69 years with an accompanying 25% RRR would result in a decline in the all-cause mortality rate of almost 3.2% and 2.6% in the UK (England & Wales) and Germany (Table, eTable 2).
For all the diseases discussed here, there were hardly any changes after accounting for a potential carry-over effect of the mortality reduction to a higher age group for whom the respective screening is not provided. The maximum change in the percentage reduction in all-cause mortality due to a carry-over effect was 0.2% (Germany: prostate cancer, UK (England & Wales): breast cancer) (data not shown).
For a relative risk of 0.97, that is a relative risk reduction of 3%, related to all-cause mortality, the required study size of a screening trial with a two-sided 95% confidence interval of +/–0.01 is 596 200. For a relative risk of 0.98, the corresponding sample size is 602 346.
Discussion
We have shown that effective early detection of cancer in the age groups eligible for screening can hardly have an effect on all-cause mortality in two representative Western European populations in the 2010s. Therefore, statements on mammography screening such as “the all-cause mortality rate in the screening group is the same as that in the unscreened group”, “mammography does not save lives” (12), or “PSA screening increases harms without changing overall mortality” are incorrect as such differences can be expected to be small (2% or less) for two reasons:
The ERSPC study revealed that the prostate cancer–specific mortality rate among men aged 55–69 years over a period of 11 years decreased by 21% with PSA screening every four years (5). The all-cause mortality rates were, on the other hand, very similar (screening: 18.2 per 1000 person-years; no screening: 18.5 per 1000 person-years; mortality rate ratio 0.99, 95% CI: [0.97–1.01]). Based on these results, Schröder et al. concluded that “PSA-based screening reduces prostate cancer mortality but does not affect all-cause mortality.” Our analysis revealed that the estimated 1% decline in all-cause mortality found in the ERSPC study corresponds to a decline that can be expected based on the current all-cause mortality and prostate cancer-specific mortality rates in European populations such as the UK (England & Wales) and Germany in 2015. As only 2.9% (UK [England & Wales]) and 2.1% (Germany) of all deaths among men aged 55–69 years are due to prostate cancer, efficient prostate cancer screening can hardly influence the all-cause mortality rate.
The ERSPC study revealed that the prostate cancer–specific mortality rate among men aged 55–69 years over a period of 11 years decreased by 21% with PSA screening every four years (5). The all-cause mortality rates were, on the other hand, very similar (screening: 18.2 per 1000 person-years; no screening: 18.5 per 1000 person-years; mortality rate ratio 0.99, 95% CI: [0.97–1.01]). Based on these results, Schröder et al. concluded that “PSA-based screening reduces prostate cancer mortality but does not affect all-cause mortality.” Our analysis revealed that the estimated 1% decline in all-cause mortality found in the ERSPC study corresponds to a decline that can be expected based on the current all-cause mortality and prostate cancer-specific mortality rates in European populations such as the UK (England & Wales) and Germany in 2015. As only 2.9% (UK [England & Wales]) and 2.1% (Germany) of all deaths among men aged 55–69 years are due to prostate cancer, efficient prostate cancer screening can hardly influence the all-cause mortality rate.
Given an all-cause mortality rate of 621 and 743 per 100 000 person-years for men and women aged 55–64 years and a colorectal cancer mortality rate of 26.7 and 27.6 per 100 000 person-years within that age group in the UK (England & Wales) and Germany respectively, sigmoidoscopy screening even among 100% of eligible people cannot produce a decline in all-cause mortality by more than 1.0–1.2%. Skin cancer screening will hardly ever result in any appreciable decline in all-cause mortality as the percentage of deaths due to skin melanoma is simply too low (0.3–0.4% of all deaths among people aged 35 years or more) even in the presence of a large RRR of 50% as postulated by the SCREEN project (10).
To observe a decline in all-cause mortality among men aged 50–74 years by 3% for example, the cancer-specific mortality rate before screening for the cancer in question has to be about 110 per 100 000 person-years given a RRR of 30% (women aged 50–74 years: 69 per 100 000 person-years). However, none of the four cancers presented here has a mortality rate without screening that is in this order in the age groups eligible for screening. For ischemic heart disease, mortality rates among people aged 45–69 years are high and a hypothetical 25% RRR would result in a 3% decline in all-cause mortality in the UK (England & Wales) and Germany.
Our sample size calculations show that the study size of screening trials needs to be extremely large (half a million) in order to be able to demonstrate a reduction in all-cause mortality of 2–3% with a narrow (95%) confidence interval. As all published screening trials have sample sizes far below half a million, one cannot expect narrow confidence intervals for the RRR of all-cause mortality in screening trials. Consequently, one cannot expect ‘statistically significant’ declines in all-cause mortality. From a public health perspective, a 2% reduction in overall mortality is a relevant effect. If 100% of 50–69 year old women in Germany participated in mammography screening, the overall mortality rate would decrease from 517 per 100 000 person-years to 508 per 100 000 person-years (–1.8%) in that age group. Therefore, 9 per 100 000 deaths per year (1.8%) would be avoided in these women.
In conclusion, because the proportion of cancer deaths in all deaths in Western Europe is relatively low, cancer screening procedures can reduce all-cause mortality by only 1–3%. However, this reduction is of public health importance and clinically relevant. Furthermore, screening procedures can have a beneficial effect on non-lethal endpoints (aggressiveness of treatment, costs, etc.).