Today, I review, link to, and excerpt from Vascular Cognitive Impairment and Dementia [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Continuum (Minneap Minn). 2022 Jun 1;28(3):750-780. doi: 10.1212/CON.0000000000001124.
There are 103 similar articles in PubMed.
The above article has been cited by 24 in PubMed.
All that follows is from the above article.
- Abstract
- INTRODUCTION
- EPIDEMIOLOGY OF VASCULAR COGNITIVE IMPAIRMENT AND DEMENTIA
- PATHOGENESIS OF VASCULAR COGNITIVE IMPAIRMENT AND DEMENTIA
- LARGE-SCALE COGNITIVE AND BEHAVIORAL NETWORKS
- CLINICAL APPROACH
- OTHER CONSIDERATIONS
- MANAGEMENT
- CONCLUSION
- ACKNOWLEDGMENT
- Footnotes
- REFERENCES
Abstract
Purpose of review: This article gives a broad overview of vascular cognitive impairment and dementia, including epidemiology, pathophysiology, clinical approach, and management. Emphasis is placed on understanding the common underlying types of cerebrovascular disease (including atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy) and awareness of rare inherited cerebrovascular disorders.
Recent findings: The pathophysiology of vascular cognitive impairment and dementia is heterogeneous, and the most recent diagnostic criteria for vascular cognitive impairment and dementia break down the diagnosis of major vascular dementia into four phenotypic categories, including subcortical ischemic vascular dementia, poststroke dementia, multi-infarct dementia, and mixed dementia. Control of cardiovascular risk factors, including management of midlife blood pressure, cholesterol, and blood sugars, remains the mainstay of prevention for vascular cognitive impairment and dementia. Cerebral amyloid angiopathy requires special consideration when it comes to risk factor management given the increased risk of spontaneous intracerebral hemorrhage. Recent trials suggest some improvement in global cognitive function in patients with vascular cognitive impairment and dementia with targeted cognitive rehabilitation.
Summary: Thorough clinical evaluation and neuroimaging form the basis for diagnosis. As vascular cognitive impairment and dementia is the leading nondegenerative cause of dementia, identifying risk factors and optimizing their management is paramount. Once vascular brain injury has occurred, symptomatic management should be offered and secondary prevention pursued.
Copyright © 2022 American Academy of Neurology.
INTRODUCTION
Dementia is defined as a decline in cognitive function causing impairment that interferes with independence in everyday activities.1 Vascular cognitive impairment and dementia refers to cognitive impairment or dementia that results from vascular brain injury. Vascular brain injury refers to damage to brain parenchyma resulting most commonly from ischemia, infarction, and hemorrhage.
Vascular cognitive impairment and dementia are among the most common causes of dementia after Alzheimer disease (AD).2 The most common risk factors for vascular cognitive impairment and dementia are cardiovascular risk factors, including hypertension, hyperlipidemia, type 2 diabetes mellitus, smoking, and atrial fibrillation.
This article explores vascular brain injury as it relates to and serves as a precursor for vascular cognitive impairment and ultimately vascular dementia and discusses the most recent evidence regarding epidemiology, pathophysiology, clinical approach, and management of vascular cognitive impairment and dementia.
EPIDEMIOLOGY OF VASCULAR COGNITIVE IMPAIRMENT AND DEMENTIA
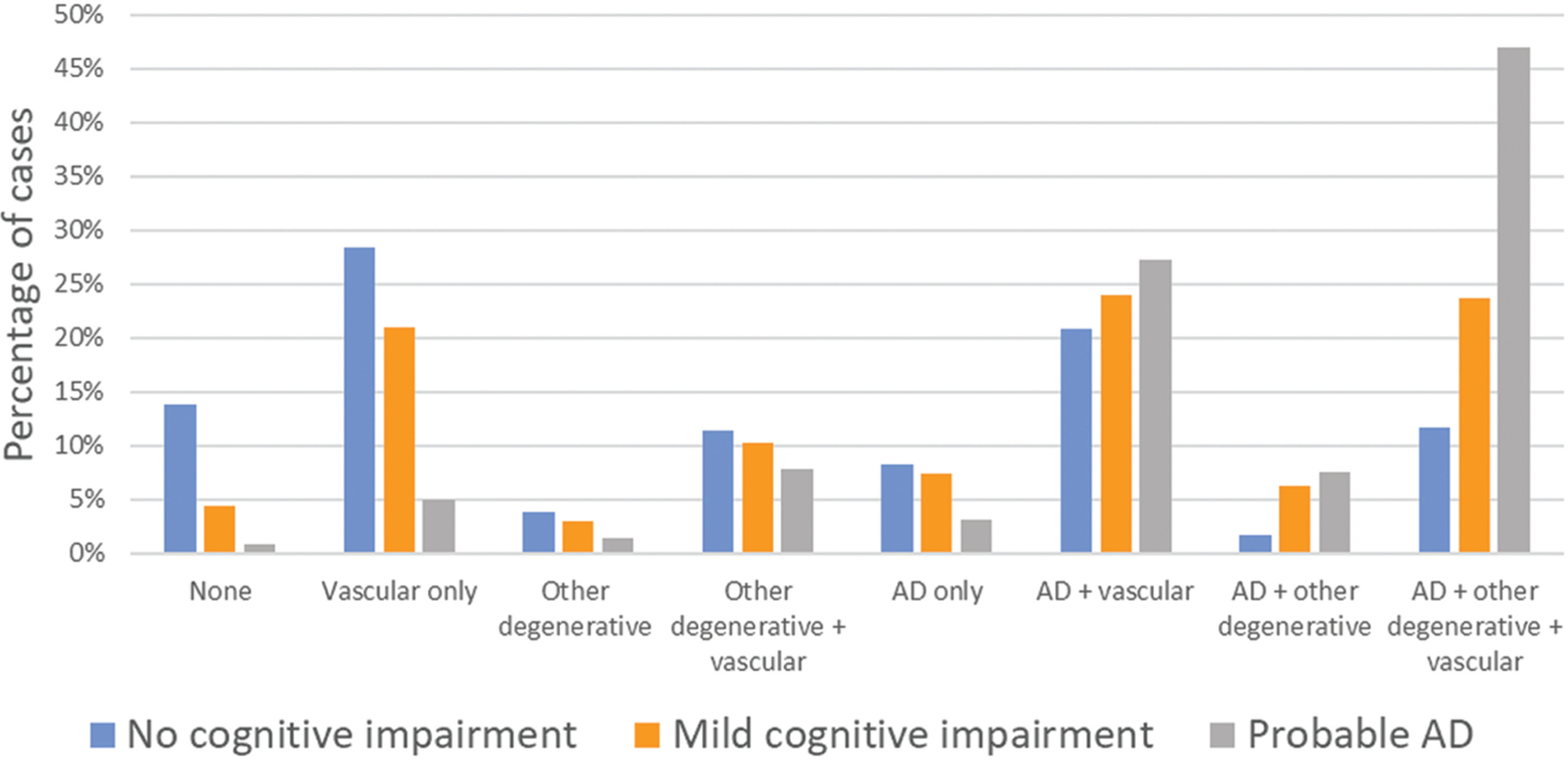
Vascular dementia is traditionally thought to be the second most common cause of dementia in the United States, comprising approximately 15% to 20% of clinically diagnosed dementia cases in North America and Europe.4 In Asia and some developing countries, the estimated burden of vascular dementia is thought to be closer to 30%,6,7 and as such, it may be the leading cause of dementia in those countries. The picture becomes more complex when neuropathologic changes form the basis of diagnosis. In some neuropathologic studies, mixed vascular and Alzheimer pathology has been found to have a prevalence of 20% to 27%,8–12 whereas others have shown a prevalence of up to 38%, with pure vascular pathology seen only in 12%.13 The probability of mixed dementia increases with increasing age, and the combination of multiple pathologies has been shown to be significantly correlated with the risk of clinical dementia.12–15 In the Rush Religious Orders Study and Rush Memory and Aging Project, neuropathologic evidence of vascular disease alone or in combination with AD was present in a plurality of cases across the spectrum of cognitive impairment, from none to major.14 However, an increasing prominence of Alzheimer pathology strongly aligns with increasing severity of cognitive impairment and dementia (FIGURE 5-1). It should be noted that cerebral amyloid angiopathy (CAA) can cause vascular brain injury as well.
FIGURE 5–1.
Prevalence of different neuropathologies among the participants in the Rush Religious Orders Study/Memory and Aging Project (ROS/MAP) who clinically had no cognitive impairment, mild cognitive impairment, or probable Alzheimer disease (AD) (n = 1078). “Other degenerative” refers to other neurodegenerative pathology, including Lewy body, transactive response DNA-binding protein 43 (TDP-43) and hippocampal sclerosis. Notably, when compiled, mixed pathology increases from about 46% of participants with no cognitive impairment to 89.7% of participants with probable AD.
a Data from Kapasi A, et al, Acta Neuropathologica.14
PATHOGENESIS OF VASCULAR COGNITIVE IMPAIRMENT AND DEMENTIA
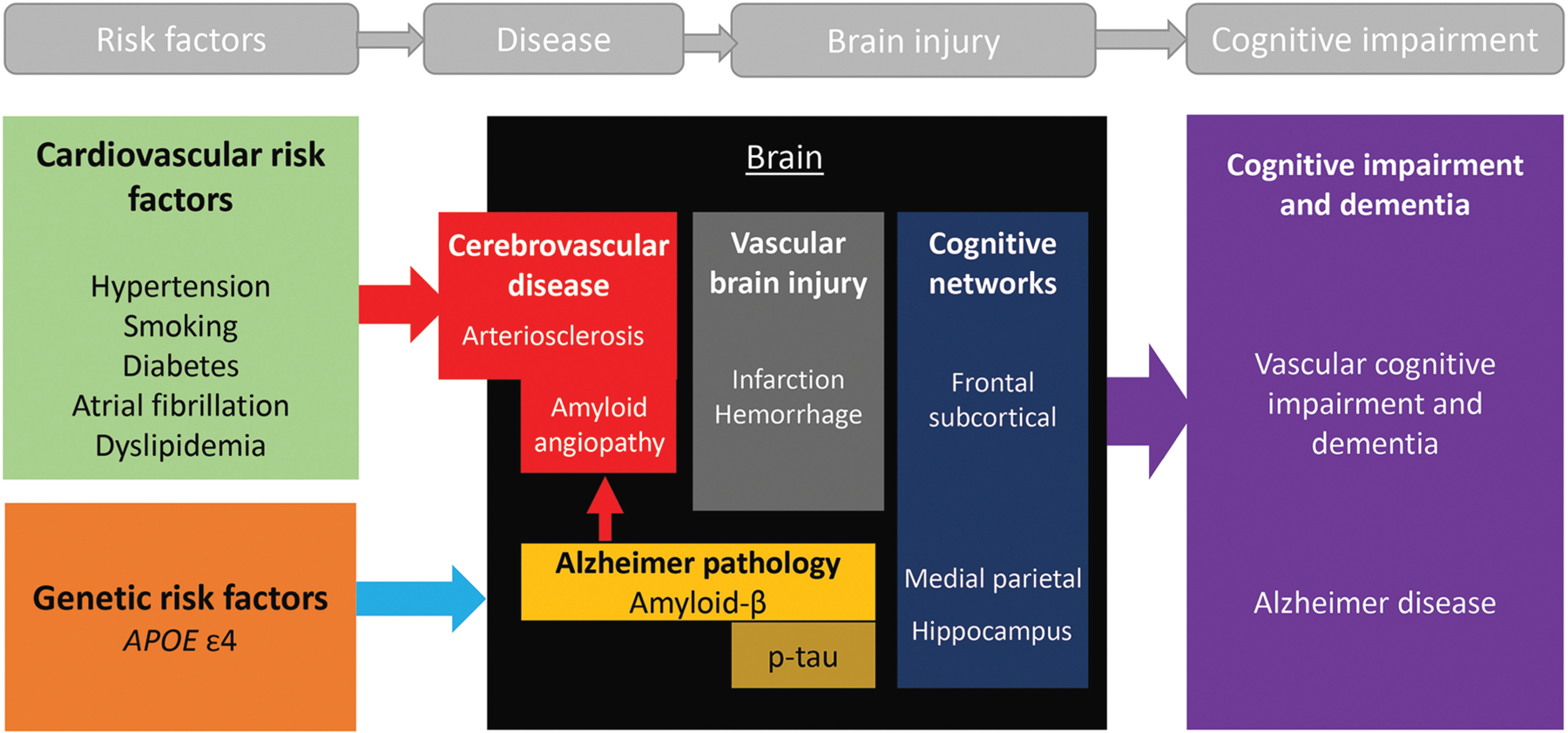
The pathogenesis of vascular cognitive impairment and dementia may be proposed as follows: vascular risk factors lead to cerebrovascular disease that results in vascular brain injury, and disruption of cognitive networks due to vascular brain injury leads to vascular cognitive impairment and dementia (FIGURE 5-2). The best treatment of vascular cognitive impairment and dementia is through prevention by detecting and mitigating vascular risk factors as early in life as possible.
FIGURE 5–2.
Pathogenesis of vascular cognitive impairment and dementia and mixed Alzheimer disease/vascular cognitive impairment and dementia. Cardiovascular risk factors are the leading cause of atherosclerotic cardiovascular disease (ASCVD), which results in cerebral vascular disease and, uncontrolled, may result in vascular brain injury and ultimately vascular cognitive impairment and dementia. Apolipoprotein E4 (APOE ε4) is associated with Alzheimer disease (AD) and cerebral amyloid angiopathy (CAA)* and is a frequent cause of mixed AD/vascular cognitive impairment and dementia. Vascular brain injury can result in damage to the frontal subcortical circuits, whereas AD tends to affect the medial parietal and hippocampal regions.
*Cerebral amyloid angiopathy: an update [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. J Neurol. 2023 May;270(5):2809-2811. doi: 10.1007/s00415-023-11631-3. Epub 2023 Mar 24.
Vascular Risk Factors
Cardiovascular risk factors increase the relative risk of dementia, which can be modified by early implementation of lifestyle changes and medications.16 For example, nearly 10% of the weighted population attributable risk for dementia is accounted for by four vascular risk factors: smoking (5.2%), hypertension (1.9%), diabetes mellitus (1.2%), and physical inactivity (1.6%). The prevalence of cardiovascular risk factors may differ significantly based on race. In the Atherosclerosis Risk in Communities Study, the prevalence of hypertension (56% versus 27%) and type 2 diabetes mellitus (18% versus 7%) was twice as high among Black people than White people.17,18 However, the strengths of association between the severity of cardiovascular risk factors and resulting vascular brain injury or vascular cognitive impairment and dementia were similar across the two racial groups. Similar correlations were found between systolic blood pressure and volume of white matter hyperintensities19 and between baseline hemoglobin A1c and subsequent cognitive decline.20 These findings suggest that disparities in the prevalence of risk factors may be related to social determinants of health more than race and that management of risk factors is equally important across racial groups. For more information on health disparities and social determinants of health, refer to the article “Health Disparities in Dementia” by Joyce (Joy) E. Balls-Berry, PhD, MPE, and Ganesh M. Babulal, PhD, OTD, MSCI, MOT, in this issue of Continuum.21
APOE ε4 predisposes to the accumulation of amyloid-β in cerebral blood vessels (resulting in CAA [Cerebral Amyloid Angiopathy]) as well as in the brain parenchyma (amyloid plaques) and is another risk factor for vascular brain injury, but it is not a risk factor for coronary artery disease. Thus, when enumerating cerebrovascular risk factors, one must think beyond traditional cardiovascular risk factors.
Major Types of Cerebrovascular Disease
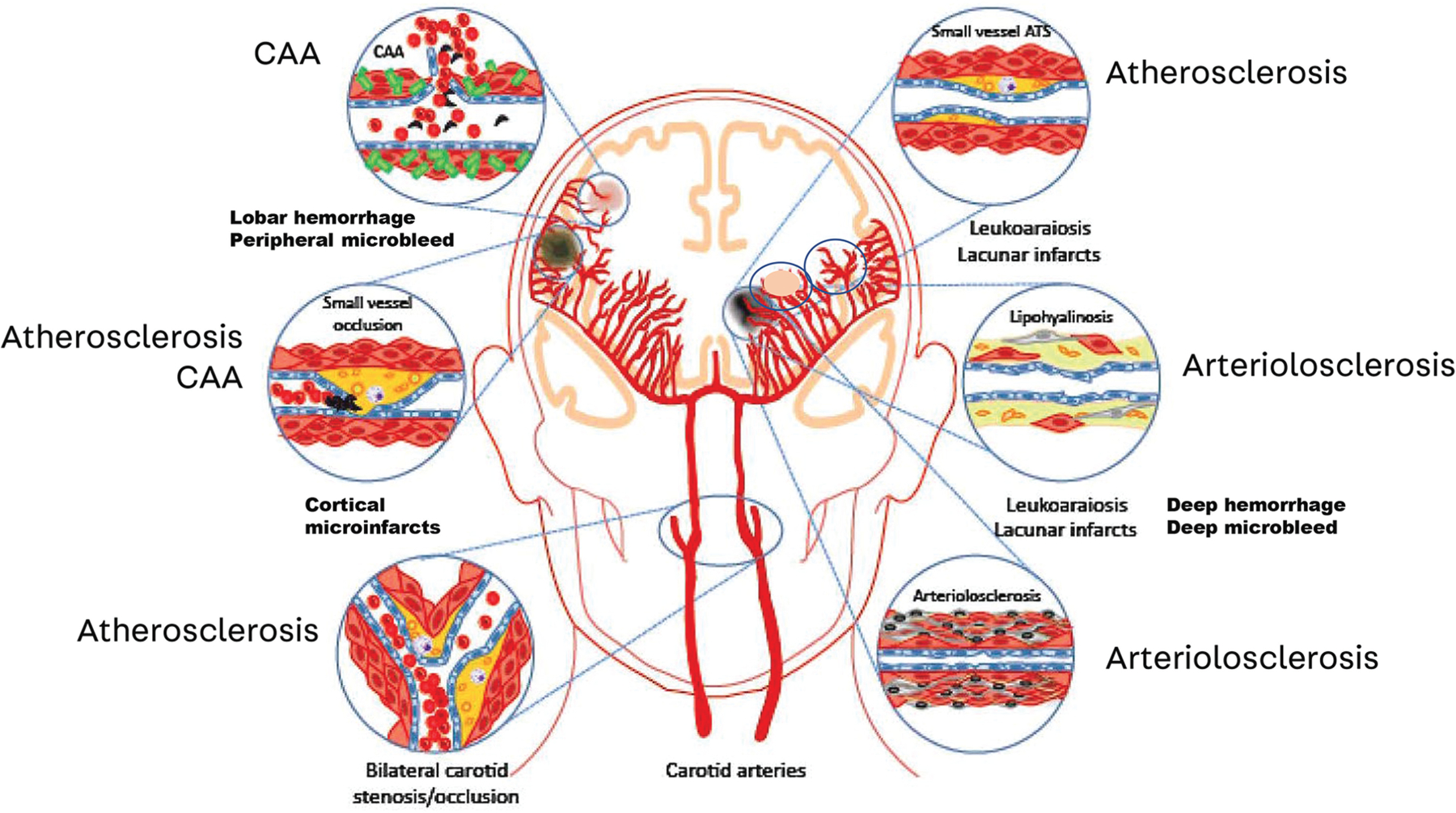
Many types of cerebrovascular diseases contribute to vascular cognitive impairment and dementia (FIGURE 5-3),22 but only the major types are discussed in this article. Specific cardiovascular risk factors and types of cerebrovascular disease show predilections for certain parts of the vascular tree (eg, large arteries, small arteries, veins, or capillaries) and are described below. These generalizations are helpful in differential diagnosis but should be considered only as general guidelines. Neuropathologic studies illustrate how various types of cerebrovascular disease often coexist and can affect multiple sites in the vascular tree.23 The past several decades have witnessed significant advances in our ability to detect vascular brain injury and the presence of cerebrovascular disease on structural and susceptibility-weighted imaging (SWI) studies.
FIGURE 5–3.
The different forms of cerebral vascular disease that may contribute to vascular cognitive impairment and dementia.
ATS = atherosclerosis; CAA = cerebral amyloid angiopathy.
Modified with permission from Iadecola C, Neuron.22 © 2013 Elsevier Inc.
*Brain Arteriolosclerosis [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Acta Neuropathol. 2021 Jan;141(1):1-24. doi: 10.1007/s00401-020-02235-6. Epub 2020 Oct 24.
ATHEROSCLEROSIS.
Atherosclerosis affects the intimal lining of large feeding arteries (eg, aorta; carotid arteries; vertebral arteries; and the pial vessels of the anterior, middle, and posterior cerebral arteries). Major risk factors for atherosclerosis include smoking and hyperlipidemia. Occlusion of large arteries by thrombosis or cardioembolism leads to wedge-shaped infarcts affecting both cortical gray and underlying subcortical white matter, thereby disrupting widespread components of multiple brain networks, resulting in stepwise functional decline and the well-recognized syndromes of multi-infarct dementia or poststroke dementia. On the other hand, artery-to-artery emboli can also result in small cortical microinfarcts, which can only be visualized with high-field MRI or postmortem.
ARTERIOLOSCLEROSIS.
Arteriolosclerosis affects the medial smooth muscle wall of small cortical and penetrating arterioles that feed the basal ganglia and deep white matter. Hypertension is the major risk factor for arteriolosclerosis. Occlusion of small arteries leads to lacunar infarcts as well as central hemorrhages and microbleeds. Widespread stenosis of the long penetrating arterioles leads to chronic ischemia of the periventricular and deep white matter, visualized as white matter hyperintensities on MRI and white matter changes that appear hypodense on CT. Lacunar infarcts and white matter hyperintensities can often be strategically located to disrupt frontal-subcortical circuits and cause impairments in executive function, recognized as the syndrome of subcortical ischemic vascular dementia or small vessel disease, described later in this article.
CEREBRAL AMYLOID ANGIOPATHY.
CAA involves buildup of amyloid-β predominantly affecting pial and cortical arteries as well as capillaries, with relative sparing of the penetrating arterioles that supply the basal ganglia (striatum and thalamus). Consensus is lacking as to whether to classify AD with CAA-related vascular brain injury as pure AD or mixed AD/vascular cognitive impairment and dementia; in this article, APOE ε4 and CAA are considered as risk factors for vascular cognitive impairment and dementia. The APOE ε4 genotype is the major risk factor for CAA. CAA is associated with lobar hemorrhages (large and small), cortical microinfarcts, and white matter hyperintensities. Although these vascular brain injury hallmarks can be detected by MRI and form the basis for a clinical diagnosis of CAA, definitive diagnosis of CAA is based on autopsy findings.
MICROVASCULAR DISEASE.
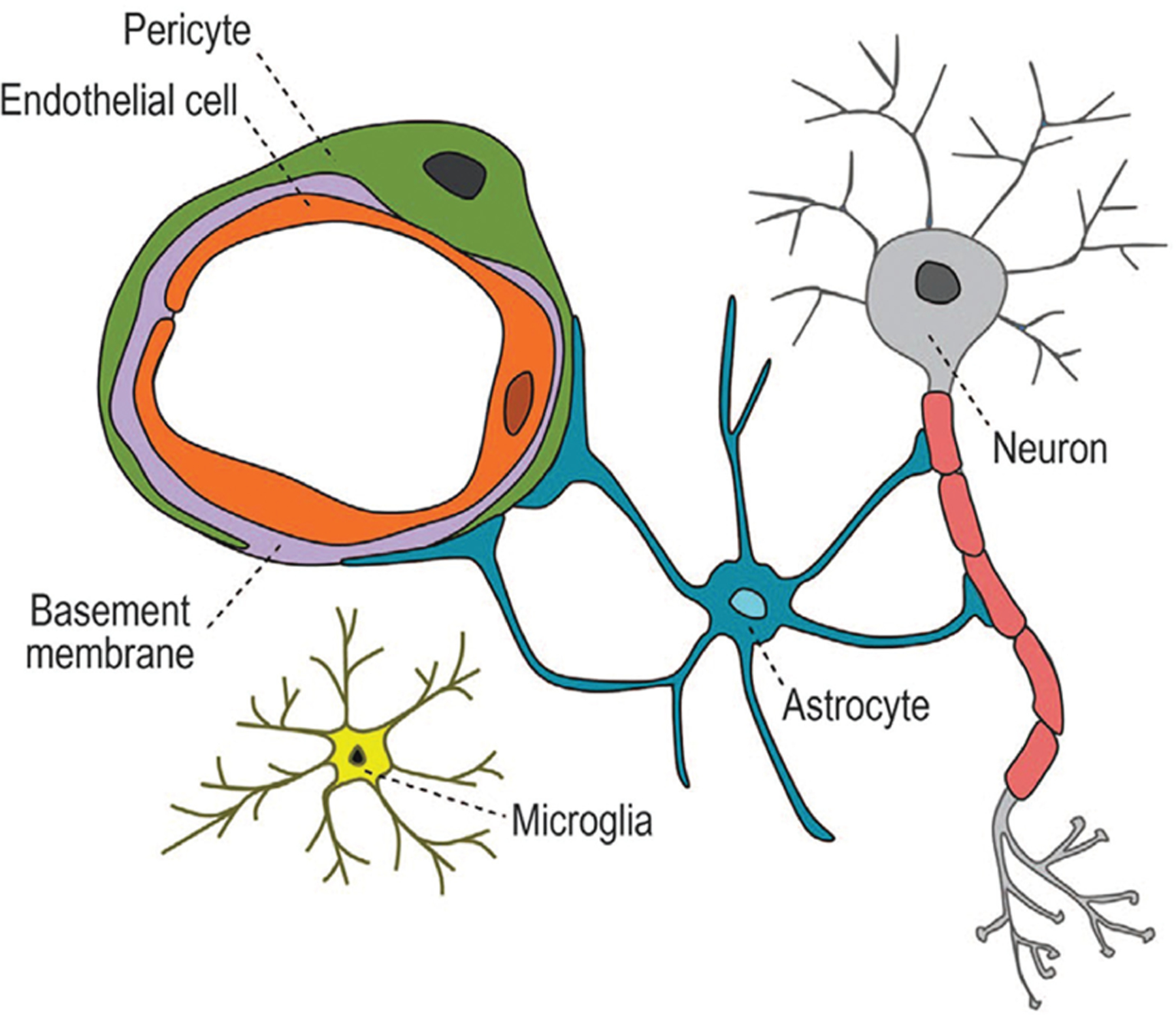
Microvascular disease involves the tissue-level delivery of fuel/nutrients and clearing of metabolic waste products that occurs at the capillary neurovascular unit (FIGURE 5-4).24–27 Type 2 diabetes mellitus is among the most common risk factors for microvascular disease. Studies by Moran and colleagues28 show that type 2 diabetes mellitus is associated with cerebral atrophy.
FIGURE 5–4.
The parts of the neurovascular unit.
Reprinted with permission from Sweeney MD, et al, J Cereb Blood Flow Metab.24 © 2015 SAGE Publications.
Regional assessment of the blood-brain barrier using dynamic contrast enhancement has been a relatively recent development.29 Increased Ktrans, a measure of leakage of gadolinium contrast from blood to brain, has been demonstrated in carriers of the APOE ε4 genotype30,31 and persons with confluent subcortical leukoencephalopathy.32,33
Evidence From Neuropathology
Neuropathologic studies illustrate a high degree of heterogeneity in the types of cerebrovascular disease and vascular brain injury and their overlap with AD pathology. In a study of patients diagnosed clinically with probable AD, 21% of participants were found to have cerebrovascular disease without AD pathology, with about 50% of these participants having both infarcts (micro and macro) and vessel disease (including atherosclerosis, arteriolosclerosis, and CAA).14 Another study of neuropathologic correlates in a population-based study showed that among participants with dementia, 42% had cerebral infarcts, 46% had cortical microvascular lesions, 38% had subcortical microvascular lesions, and 42% had some amount of CAA, although the proportion of participants with overlaps in pathology was not explicitly stated.34
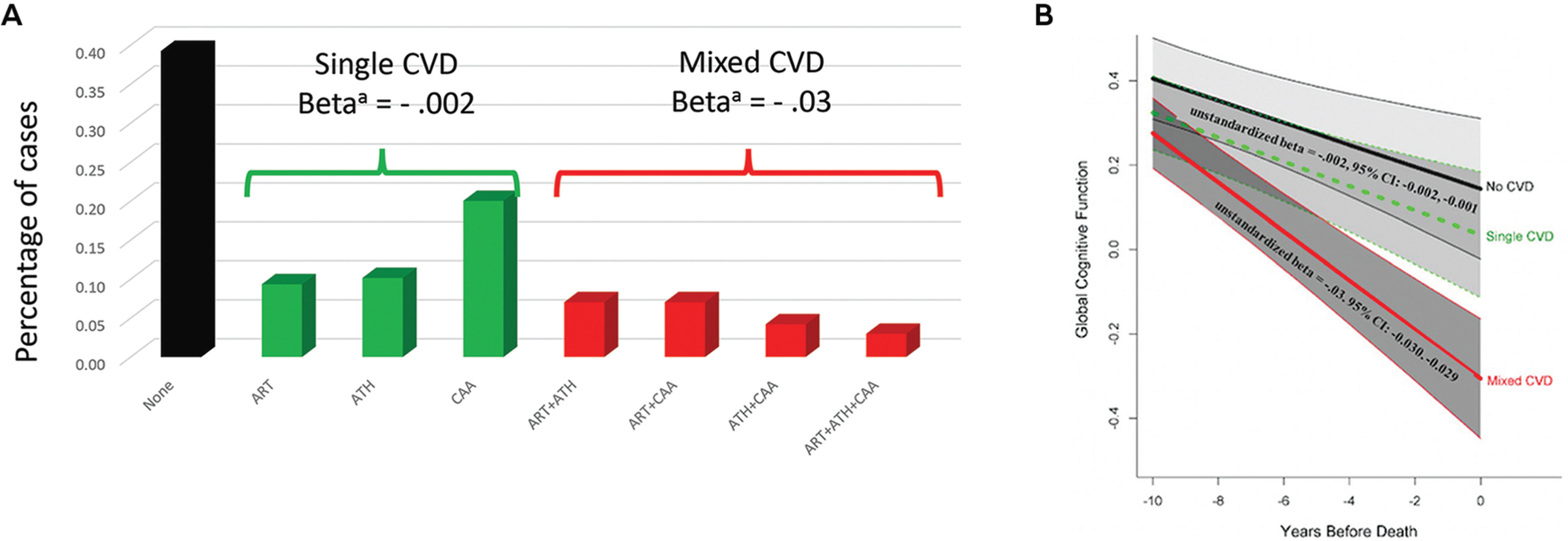
A large combined autopsy series from the Rush Religious Orders Study, Rush Memory and Aging Project, and the Minority Aging Research Study (n = 1474) examined the frequency of the major types and combinations of cerebrovascular disease and their associations with longitudinal cognitive decline (FIGURE 5-5).35 Pure arteriolosclerosis and atherosclerosis without brain tissue injury (defined as macroinfarcts or microinfarcts) were associated with little overall decline in domain-specific cognitive function, whereas CAA was associated with decline in semantic memory and visuospatial ability. On the other hand, mixed types of vascular disease (eg, various combinations of arteriolosclerosis, atherosclerosis, and CAA) were strongly associated with cognitive decline (β = −0.03, standard error = .007, P<.001), more so if evidence existed of vascular brain injury.
FIGURE 5–5.
The association of single (green) and mixed (red) cerebrovascular disease (CVD) profile groups with global cognitive decline compared with participants with no cerebrovascular disease (black). In an autopsy series on participants with Alzheimer disease from the Rush Religious Orders Study, Rush Memory and Aging Project, and the Minority Aging Research Study, the major types of cerebrovascular disease (CVD) and their various combinations were examined. Notably, mixed CVD produced greater cognitive decline over time when compared to single or no CVD groups.
a Unstandardized beta for rate of cognitive decline for single CVD and mixed CVD compared to reference group with no CVD.
ART = arteriolosclerosis; ATH = atherosclerosis; CAA = cerebral amyloid angiopathy.
Panel B reprinted with permission from Lamar M, et al.35 © 2021 American Heart Association, Inc.
LARGE-SCALE COGNITIVE AND BEHAVIORAL NETWORKS
With the advent of structural and functional neuroimaging, significant advances have been made in delineating large-scale human brain networks.36 Network-sensitive neuroimaging methods have shown how different neurodegenerative syndromes (eg, Alzheimer dementia, semantic dementia, and behavioral variant frontotemporal dementia) cause circumscribed atrophy within distinct intrinsic functional connectivity networks for memory, language, and behavior.37 Similar network analyses elucidate how vascular brain injury disrupts brain networks (eg, cognitive control, default mode, salience networks), resulting in cognitive and behavioral changes following stroke (eg, executive dysfunction,38 depression, and apathy39).
The anatomic signature of vascular brain injury is highly heterogeneous, although patterns are discernable. For example, fluent and nonfluent aphasia are associated with infarctions in the territory of the left middle cerebral artery. Impairment of executive function is commonly associated with subcortical lacunar infarcts and confluent white matter changes.
A series of parallel frontal-subcortical anatomic circuits are important in modulating behavior.40 The dorsolateral prefrontal circuit regulates central executive control, including anticipating, planning, and monitoring performance. Disruption along this pathway may result in impairments of cognitive testing, including poor attention and set shifting consistent with dysexecutive function. Two of the frontal-subcortical circuits mediate behavior: the anterior cingulate circuit mediates motivation (CASE 5-1), and the orbitofrontal circuit is involved in the salience network and mediates inhibition.
CASE 5–1
A 76-year-old man with a past medical history of hypertension and hyperlipidemia presented with a 2-year history of short-term memory decline. His family had noted changes in his personality as well, with decreased initiation. He was no longer engaged and interested in his family’s affairs, including spending time with his grandchildren, which he previously enjoyed. He appeared to be generally apathetic. He could bathe and dress himself but required repeated reminders and encouragement from his family to do so. His responses and movements were slowed.
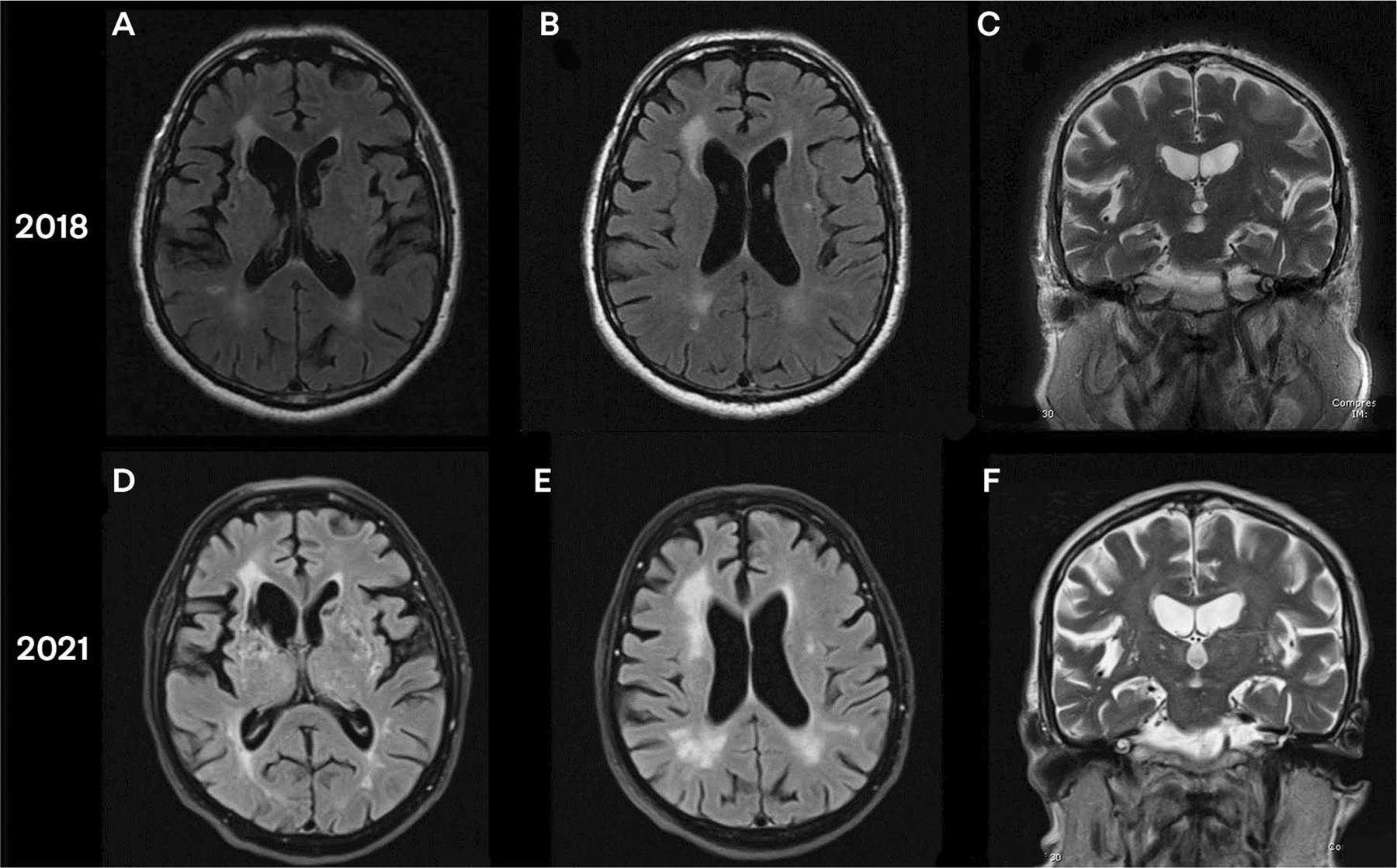
On mental status examination, he scored 19/30 on the Montreal Cognitive Assessment (MoCA), with noted attentional deficits (unable to do digits forward or backward) and poor memory (with 0/5 words recalled, which improved to 3/5 with category cues). On the remainder of his neurologic examination, he was noted to have slightly increased tone in bilateral upper extremities and slightly decreased right arm swing when walking. An MRI was obtained, which showed multiple lacunar infarcts involving the frontal-subcortical circuits bilaterally, with sparing of the hippocampi. (FIGURE 5-6).
FIGURE 5–6.
Imaging of the patient in CASE 5-1. A, Axial fluid-attenuated inversion recovery (FLAIR) MRI shows lacunar infarct of bilateral caudate and right external capsule from initial presentation. B, Axial FLAIR MRI shows white matter disease (Fazekas scale grade 2) from initial presentation. C, Coronal T2-weighted MRI shows hippocampal sparing from initial presentation. D, Axial FLAIR MRI shows progression of white matter disease and lacunar infarcts on left basal ganglia as well as adjacent to right anterior horn, 3 years later. E, Axial FLAIR MRI shows white matter disease (Fazekas scale grade 3), 3 years later. F, Coronal T2-weighted MRI shows continued hippocampal sparing, despite progression in generalized atrophy (slight enlargement of ventricles can be seen), 3 years later.
COMMENT
This patient’s MRI showed multiple lacunar infarcts bilaterally (FIGURE 5-6A). Of note, multiple infarcts can be seen in the bilateral striatum, resulting in disruption of the frontal-subcortical circuits and thus the patient’s loss of motivated behavior, as well as disruption of motor pathways of the basal ganglia, resulting in vascular parkinsonism. His white matter disease is grade 2 on the Fazekas scale (described later in this article) (FIGURE 5-6B). Bilateral sparing of the hippocampi is seen (FIGURE 5-6C). On follow-up MRI 3 years later, with continued poor cardiovascular risk factor control, progression of white matter disease (FIGURES 5-6D and 5-6E) and unchanged relative sparing of bilateral hippocampi are seen (FIGURE 5-6F).
CLINICAL APPROACH
The mainstay of the clinical approach to vascular cognitive impairment and dementia remains the history and physical/neurologic (including mental status) examination. Imaging lends significant sensitivity and specificity to the etiologic diagnosis. The location of vascular brain injury goes a long way in explaining signs and symptoms. Vascular risk factors and subtype of cerebrovascular disease are especially relevant for designing primary and secondary prevention strategies.
Clinical History
As with all neurologic disorders, obtaining a careful history can provide significant insight into the cause of the disease. For neurocognitive disorders, understanding the initial presenting symptom is key to diagnosis, and the same holds true for diagnosing vascular cognitive impairment and dementia. As Alzheimer disease and vascular cognitive impairment and dementia are among the top two causes of dementia, a quick reference for distinguishing key aspects of the two is provided in TABLE 5-1.
TABLE 5–1.
Clinical Comparison of Vascular Cognitive Impairment and Dementia and Alzheimer Disease
Evaluation Vascular cognitive impairment and dementia Alzheimer disease History History of present illness Multi-infarct/poststroke: stepwise (temporal relationship)
Subcortical ischemic vascular disease: slowly progressiveSlowly progressive Past medical/family history Cerebrovascular disease, coronary artery disease, peripheral vascular disease Alzheimer disease Mental status Multi-infarct/poststroke: location-dependent
Subcortical ischemic vascular disease: dysexecutive functionAmnestic-type memory impairment (poor cueing, rapid forgetting) Physical examination Neurologic Focal signs, gait disturbance Normal neurologic examination other than mental status (until later stages) Cardiovascular Changes consistent with cardiovascular risk factors (retinal, carotid, peripheral artery disease), high blood pressure Normal Laboratory tests Elevated hemoglobin A1c, lipids; check ECG for atrial fibrillation
Routine laboratory tests for reversible dementia (comprehensive metabolic panel, complete blood cell count, thyroid-stimulating hormone [TSH], vitamin B12 level, fluorescent treponemal antibody absorption) should be negativeRoutine laboratory tests for reversible dementia (comprehensive metabolic panel, complete blood cell count, thyroid-stimulating hormone [TSH], vitamin B12 level, fluorescent treponemal antibody absorption) should be negative Structural imaging MRI/CT Focal infarcts in strategic location, confluent white matter changes, generalized atrophy Hippocampal, temporal, parietal, and frontal atrophy Metabolic imaging FDG-PET Multifocal hypometabolism depending on areas of vascular brain injury Hypometabolism in parietal, temporal, and frontal lobes with sparing of primary motor-sensory cortex Biomarkers None CSF or PET amyloid-β or phosphorylated tau CSF = cerebrospinal fluid; CT = computed tomography; ECG = electrocardiogram; FDG-PET = fludeoxyglucose positron emission tomography; MRI = magnetic resonance imaging; PET = positron emission tomography.
A past medical history of multiple cardiovascular risk factors and a surgical history of cardiac surgeries or procedures for peripheral vascular disease may raise concern for vascular brain injury. Initial cognitive symptoms of subtle changes in executive function and attention rather than memory impairment would be expected in subcortical ischemic vascular dementia. A chief complaint of sudden onset of cognitive changes in conjunction with symptoms of a stroke would be consistent with poststroke dementia. A family history of dementia may be uncovered, particularly in the presence of cardiovascular risk factors. Social history may reveal many pack-years of smoking. Although neuroimaging is helpful in seeing evidence of vascular brain injury suggestive of the diagnosis of vascular cognitive impairment and dementia, it may not always be available or reliable, and the importance of the history should not be overlooked.
Physical and Neurologic Examination
When evaluating for vascular cognitive impairment and dementia, a careful physical examination can reveal many significant findings related to cardiovascular risk factors. Funduscopic examination may reveal hypertensive retinopathy. Examination of the extremities, particularly the legs, may reveal the skin changes of peripheral vascular disease or pitting edema from heart failure. A thorough cardiopulmonary examination may reveal an irregular rhythm concerning for atrial fibrillation, a carotid bruit indicating large vessel plaque buildup, or crackles from fluid overload due to heart failure.
Neurologic findings can vary widely from focal neurologic deficits consistent with stroke (eg, facial droop, visual field cut, hemiplegia, hemisensory loss) to evidence of peripheral neuropathy indicating poorly controlled diabetes. Parkinsonism due to vascular brain injury may mimic idiopathic Parkinson disease, with bradykinesia, gait disturbance, and rigidity.
Cognitive Examination
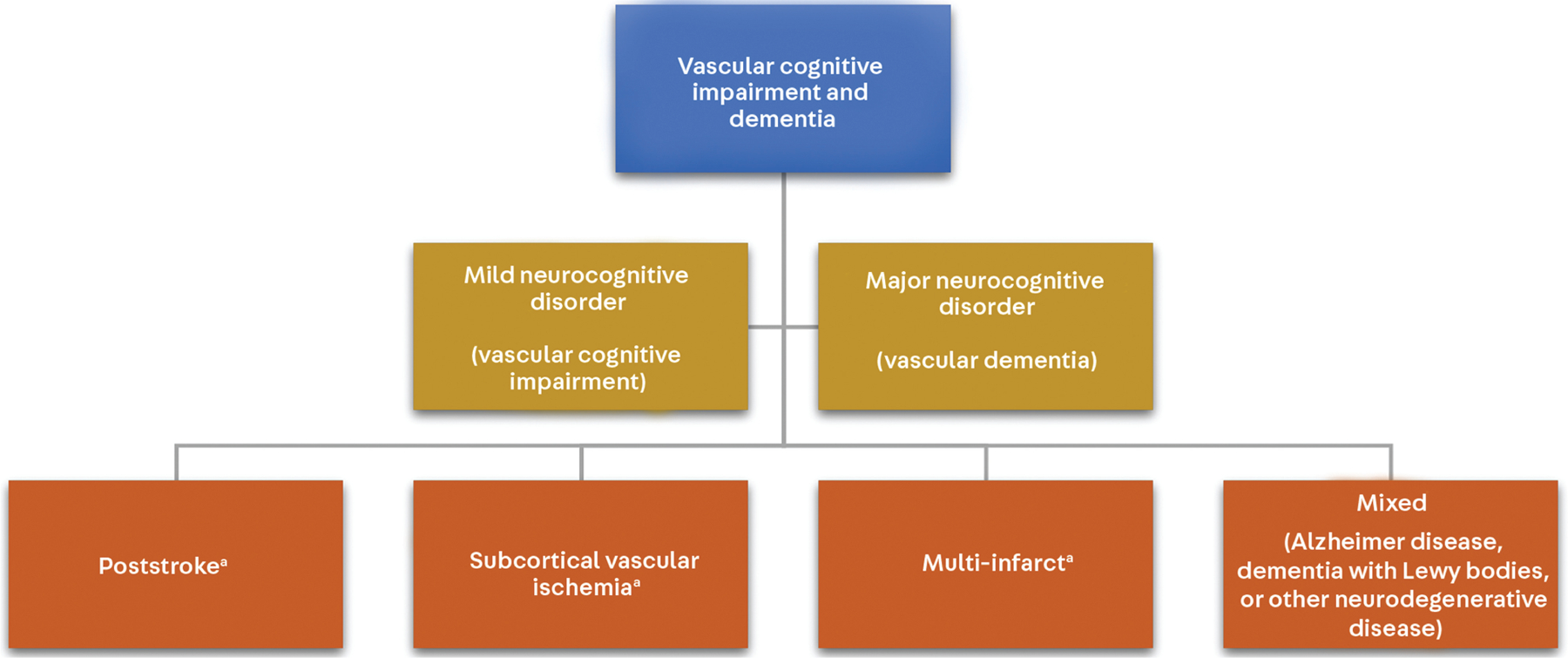
The pattern of cognitive impairment in vascular cognitive impairment and dementia can vary widely depending upon the location of vascular brain injury. The VICCCS (Vascular Impairment of Cognition Classification Consensus Study) criteria delineated four phenotypic subgroups, as discussed below (FIGURE 5-7).40
FIGURE 5–7.
The VICCCS (Vascular Impairment of Cognition Classification Consensus Study) criteria for vascular cognitive impairment and dementia.
a Although these subtypes are listed separately from “mixed,” any subtype of vascular cognitive impairment and dementia has the potential to have mixed pathology.
Modified with permission from Skrobot O, et al, Alzheimers Dement.41 © 2016 The Alzheimer’s Association.
SUBCORTICAL ISCHEMIC VASCULAR DEMENTIA.