In this post, I list and link to the mental health measures used to measure treatment response to the novel anti-depressant, Auvelity, in the GEMINI trial.* And I link to the QuickSCID-5. I will be posting about my method for diagnosing and monitoring psychiatric problems in an upcoming post.
*Efficacy and Safety of AXS-05 (Dextromethorphan-Bupropion) in Patients With Major Depressive Disorder: A Phase 3 Randomized Clinical Trial (GEMINI) [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. J Clin Psychiatry. 2022 May 30;83(4):21m14345
Instruments used:
- Montgomery-Asberg Depression Rating Scale (MADRS)
- Clinician Global Impression-Severity (CGI-S)
- Structured Clinical Interview
- Note to me: I have a print copy of SCID-5-CV.
- Please see and review QuickSCID-5
- I purchased a copy of QuickSCID-5 on 9-21-2022
- “A fully structured diagnostic instrument that employs interview questions adapted from the Structured Clinical Interview for DSM-5 (SCID-5). The QuickSCID-5 is a briefer, more time-efficient version of the SCID designed to be administered usually in 30 minutes or less.”
- “The shorter administration time results from the fact that QuickSCID-5 consists almost entirely of closed-ended questions that can be answered YES or NO by the patient, dispensing with the requirement in the standard SCID that the interviewer elicit descriptive examples and ask enough follow-up questions until the interviewer has enough information to determine whether the DSM-5 diagnostic criteria are met. Consequently, unlike the standard SCID, which requires the interviewer to have specialized training in diagnostic interviewing, QuickSCID can be administered by any clinician, including those in training. Other features include a visually appealing format and easy to follow skip instructions.”
- “QuickSCID has a modular design so that only modules of interest need be administered.”
- Structured Clinical Interview for DSM Disorders (SCID) SCID-5-CV
- FAQ
- NetSCID-5 User Guide-“web-based version of the Structured
Clinical Interview for DSM-5 (SCID-5).”
- Patient Global Impression-Improvement (PGI-I)
- Quick Inventory of Depressive Symptomatology-Self-Rated (QIDS-SR-16)
- Sheehan Disability Scale (SDS)
- The Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF)
- Columbia-Suicide Severity Rating Scale (C-SSRS)
- MADRS-6
- Physician Withdrawal Checklist
PATIENT POPULATION
Patients were men or women 18–65 years of age with a primary diagnosis of MDD, experiencing a major depressive episode of at least 4 weeks in duration, and having a Montgomery-Asberg Depression Rating Scale (MADRS)18 total score of 25 or higher, corresponding to moderate or greater severity, with higher scores indicating more severe depression.
Patients were also required to have a score on the Clinician Global Impression-Severity (CGI-S)19 scale of 4 or higher (range, 1 to 7, with higher scores indicating greater severity of illness).
The diagnosis of depression was established using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), criteria for MDD without psychotic features, based on the Structured Clinical Interview,20 which has been shown to diagnose MDD more conservatively than other structured interviews.
ENDPOINTS
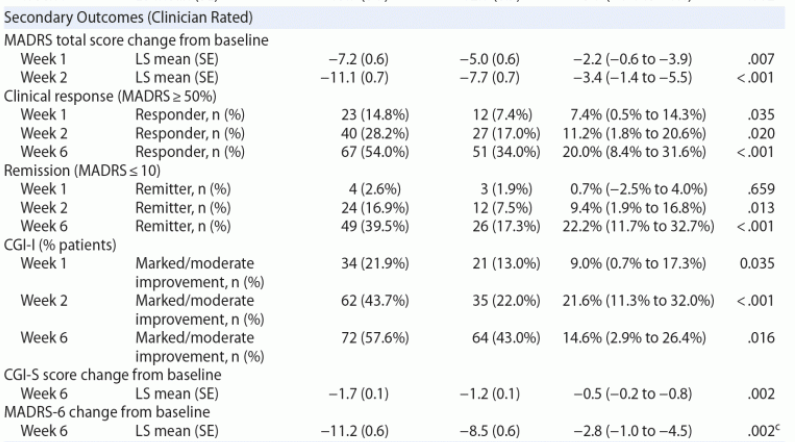
The primary endpoint was the change from baseline to week 6 in the MADRS total score. The MADRS is a 10-item clinician-rated questionnaire ranging from 0 to 60, with higher scores representing more severe depression. The key secondary endpoints were the change from baseline in the MADRS total score at week 1; change from baseline in the MADRS total score at week 2; remission, defined as MADRS total score ≤ 10, at week 2; and clinical response, defined as ≥ 50% reduction in MADRS total score, at week 6.
Other secondary endpoints included the )19; CGI-S (scores range from 1 [normal state] to 7 [among the most extremely ill])19; Patient Global Impression-Improvement (PGI-I; scores range from 1 [very much improved] to 7 [very much worse])19; Quick Inventory of Depressive Symptomatology-Self-Rated (QIDS-SR-16; scores range from 0 to 27 with higher scores representing more severe depression)22; Sheehan Disability Scale (SDS; scores range from 0 to 30 with higher scores indicating more severe disability)23; the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF; scores are based on the percentage of the maximum total score with higher percentages indicating greater satisfaction)24; and the MADRS-6 (a subscale of the 10-item MADRS evaluating the core symptoms of depression [apparent sadness, reported sadness, inner tension, lassitude, inability to feel, and pessimistic thought]).25,26
Safety was assessed based on the incidence of adverse events; changes in vital signs, clinical laboratory measurements, physical examinations, and electrocardiograms; assessment of suicidal ideation and behavior, with the use of the ; and assessment for withdrawal-related symptoms, using the Physician Withdrawal Checklist. Adverse events during the treatment period were defined as adverse events occurring from the time of administration of the first dose of dextromethorphan-bupropion or placebo until 7 days after the last dose.