In this post I review Metabolic Alkalosis with resources from emedicine.medscape.com. [and others*].
First we’re going to review a table and section from Diagnosis of metabolic acid-base disorders. September 12, 2019 by Dr. Josh Farkas of the Internet Book Of Critical Care:
getting started: metabolic vs. respiratory abnormalities
metabolic vs. respiratory pH disorders
- Metabolic disorders involve a primary change in the serum bicarbonate and/or anion gap.
- Respiratory disorders involve primary changes in the pCO2 (due to changes in CO2 removal by the lungs).
ABG/VBG isn’t needed to evaluate metabolic pH disorders
- Complete analysis of pH status requires blood gas analysis, but all you need to determine the metabolic pH disorders is an electrolyte panel.
- Analysis of the metabolic pH disorders is usually the most important component (and frequently sufficient to guide treatment).
- Metabolic pH analysis should be performed on every set of electrolytes obtained from every patient. Additional evaluation with ABG/VBG may be performed more selectively.
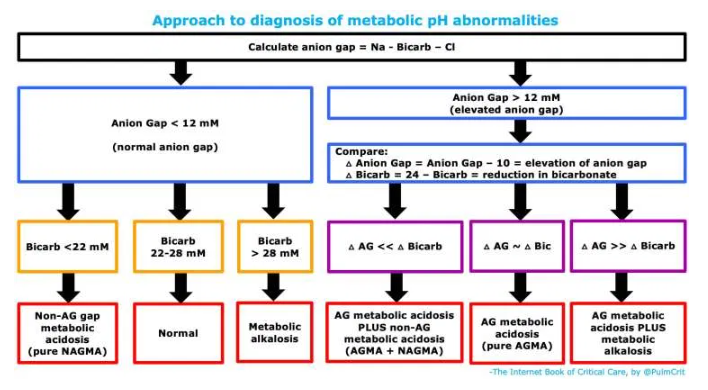
diagnostic approach to metabolic pH abnormalities
basic properties of the anion gap
- Anion Gap (AG) = Na – Bicarb – Chloride
- A normal anion gap is roughly 4-12 mM.
1. determination of the anion gap to evaluate for anion gap metabolic acidosis (AGMA)
- More on the anion gap above.
2. if the anion gap is normal, just look at the bicarbonate
- Bicarbonate <22 mM with a normal anion gap indicates a pure non-anion-gap metabolic acidosis (NAGMA).
- Bicarbonate >28 mM with a normal anion gap indicates a pure metabolic alkalosis.
- A bicarbonate of 22-28 mm with a normal anion gap indicates a normal metabolic pH status.
3. if the anion gap is elevated, determine the “delta delta”
- What are these?
- Delta anion gap = (Anion Gap) – 10. This is roughly the degree of elevation of the anion gap.
- Delta bicarbonate = 24 – bicarbonate. This is roughly the degree of reduction of the serum bicarbonate.
- Comparing these values can help determine if there is an additional process, in combination with the anion gap metabolic acidosis. Specifically:
- (1) If delta anion gap is roughly equal to the delta bicarbonate, then no other process is present. This is about what we would expect for an isolated, pure anion gap metabolic acidosis.
- (2) If the delta anion gap is much higher than the delta bicarbonate, then a second process is present which is increasing the bicarbonate level. This reveals a combination of an anion gap metabolic acidosis plus a metabolic alkalosis.
- (3) If the delta anion gap is much lower than the delta bicarbonate, then this reveals a second process which is decreasing the bicarbonate. This indicates a combined anion gap metabolic acidosis plus a non-anion-gap metabolic acidosis.
Here are excerpts from Resource (1) Metabolic Alkalosis:
Practice Essentials
Metabolic alkalosis is a primary increase in serum bicarbonate (HCO3–) concentration. This occurs as a consequence of a loss of H+ from the body or a gain in HCO3–. In its pure form, it manifests as alkalemia (pH >7.40). As a compensatory mechanism, metabolic alkalosis leads to alveolar hypoventilation with a rise in arterial carbon dioxide tension (PaCO2), which diminishes the change in pH that would otherwise occur.
Normally, arterial PaCO2 increases by 0.5-0.7 mm Hg for every 1 mEq/L increase in plasma bicarbonate concentration, a compensatory response that is very quick. If the change in PaCO2 is not within this range, then a mixed acid-base disturbance occurs. For example, if the increase in PaCO2 is more than 0.7 times the increase in bicarbonate, then metabolic alkalosis coexists with primary respiratory acidosis. Likewise, if the increase in PaCO2 is less than the expected change, then a primary respiratory alkalosis is also present.
The first clue to metabolic alkalosis is often an elevated bicarbonate concentration that is observed when serum electrolyte measurements are obtained. Remember that an elevated serum bicarbonate concentration may also be observed as a compensatory response to primary respiratory acidosis. However, a bicarbonate concentration greater than 35 mEq/L is almost always caused by metabolic alkalosis.
Metabolic alkalosis is diagnosed by measuring serum electrolytes and arterial blood gases. If the etiology of metabolic alkalosis is not clear from the clinical history and physical examination, including drug use and the presence of hypertension, then a urine chloride ion concentration can be obtained. Calculation of the serum anion gap may also help to differentiate between primary metabolic alkalosis and metabolic compensation for respiratory acidosis. (See Workup.)
The management of metabolic alkalosis depends primarily on the underlying etiology and on the patient’s volume status. Direct treatment of the alkalosis itself (eg, administration of acidic intravenous solutions) may be indicated in some cases (see Treatment).
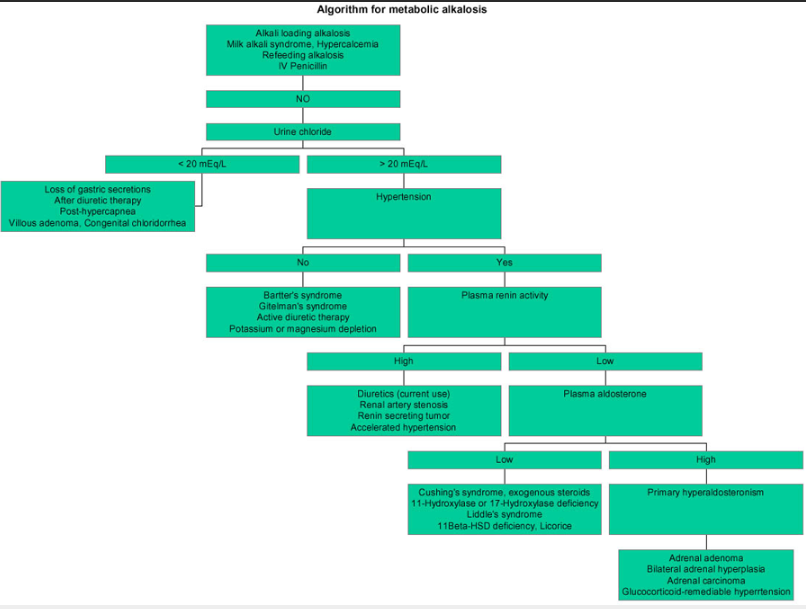
An algorithmic approach to metabolic alkalosis is depicted in the image below.
Etiology
The most common causes of metabolic alkalosis are the use of diuretics and the external loss of gastric secretions. Causes of metabolic alkalosis can be divided into chloride-responsive alkalosis (urine chloride < 20 mEq/L), chloride-resistant alkalosis (urine chloride >20 mEq/L), and other causes, including alkali-loading alkalosis.
Chloride-responsive alkalosis
The principal causes of chloride-responsive alkalosis are the loss of gastric secretions, ingestion of large doses of nonabsorbable antacids, and use of thiazide or loop diuretics. [1] Miscellaneous causes account for the remainder of cases.
Chloride-resistant alkalosis
Causes of chloride-resistant alkalosis can be divided into those associated with hypertension and those associated with hypotension or normotension. The former may result from primary hyperaldosteronism, as well as a variety of acquired and hereditary disorders. An adrenal adenoma (most common), bilateral adrenal hyperplasia, or an adrenal carcinoma may cause primary hyperaldosteronism. [3]
Other causes
The kidneys are able to excrete any excess alkali load, whether it is exogenous (eg, infusion of sodium bicarbonate) or endogenous (eg, metabolism of lactate to bicarbonate in lactic acidosis). However, in renal failure or in any condition that maintains the alkalosis, this natural ability of the kidneys to excrete the excess bicarbonate is impaired. Examples include the following:
Milk-alkali syndrome comprises hypercalcemia, renal insufficiency, and metabolic alkalosis. Before the advent of H2-receptor antagonists, milk-alkali syndrome was observed in patients who ingested large amounts of milk and antacids as treatment for peptic ulcers. Currently, the syndrome is observed mainly in people who chronically ingest large doses of calcium carbonate, with or without vitamin D (typically for osteoporosis prevention). [5]
Summary of causes of metabolic alkalosis
Causes of chloride-responsive alkalosis (urine chloride < 20 mEq/L) include the following:
Loss of gastric secretions – Vomiting, NG suction
Loss of colonic secretions – Congenital chloridorrhea, villous adenoma
Thiazides and loop diuretics (after discontinuation)
Posthypercapnia
Cystic fibrosis
Causes of chloride-resistant alkalosis (urine chloride >20 mEq/L) with hypertension include the following:
Primary hyperaldosteronism – Adrenal adenoma, bilateral adrenal hyperplasia, adrenal carcinoma, glucocorticoid-remediable hyperaldosteronism
11B-HSD2 – Genetic, licorice, chewing tobacco, carbenoxolone
CAH – 11-Hydroxylase or 17-hydroxylase deficiency
Current use of diuretics in hypertension
Cushing syndrome
Exogenous mineralocorticoids or glucocorticoids
Liddle syndrome
Renovascular hypertension
Causes of chloride-resistant alkalosis (urine chloride >20 mEq/L) without hypertension include the following:
Bartter syndrome
Gitelman syndrome
Severe potassium depletion
Current use of thiazides and loop diuretics
Hypomagnesemia
Other causes include the following:
Exogenous alkali administration – Sodium bicarbonate therapy in the presence of renal failure, metabolism of lactic acid or ketoacids
Milk-alkali syndrome
Hypercalcemia
Intravenous penicillin
Refeeding alkalosis
Massive blood transfusion
Epidemiology
Metabolic alkalosis is the most common acid-base disturbance observed in hospitalized patients, accounting for approximately 50% of all acid-base disorders.
Mortality rates have been reported as 45% in patients with an arterial blood pH of 7.55 and 80% when the pH was greater than 7.65. Prognosis also depends in part on the specific condition or circumstances that led to metabolic alkalosis.
History
Symptoms of metabolic alkalosis are not specific. Because hypokalemia is usually present, the patient may experience weakness, myalgia, polyuria, and cardiac arrhythmias.
Hypoventilation develops because of inhibition of the respiratory center in the medulla. Symptoms of hypocalcemia (eg, jitteriness, perioral tingling, muscle spasms) may be present.
The clinical history is helpful in establishing the etiology. Important points in the history include the following:
Vomiting or diarrhea – Gastrointestinal (GI) losses of hydrochloric acid (HCl)
Age of onset and family history of alkalosis – Familial disorders (eg, Bartter syndrome, which starts during childhood)
Renal failure – Alkali-loading alkalosis develops only when impairment of renal function occurs
Drug use (eg, loop or thiazide diuretics; licorice; tobacco chewing; carbenoxolone; fludrocortisone; glucocorticoids; antacids [eg, magnesium hydroxide]; calcium carbonate)
Physical Examination
The physical signs of metabolic alkalosis are not specific and depend on the severity of the alkalosis. Because metabolic alkalosis decreases ionized calcium concentration, signs of hypocalcemia (eg, tetany, Chvostek sign, Trousseau sign), change in mental status, or seizures may be present.
Physical examination is helpful to establish the cause of metabolic alkalosis. Important aspects of the physical examination include the evaluation of hypertension and of volume status.
Hypertension accompanies several causes of metabolic alkalosis (see Etiology). Volume status assessment includes evaluation of orthostatic changes in blood pressure and heart rate, mucous membranes, presence or absence of edema, skin turgor, weight change, and urine output. Volume depletion usually accompanies chloride-responsive alkalosis, while volume expansion accompanies chloride-resistant alkalosis.
Bulimia
Because patients with bulimia frequently self-induce vomiting, they may have erosions of teeth enamel and dental caries because of repeatedly exposing their teeth to gastric acid.
Cushing syndrome
Findings associated with Cushing syndrome include the following:
Obesity
Moon face
Buffalo hump
Hirsutism
Violaceous skin striae
Acne
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH): Infants with CAH secondary to 11-hydroxylase deficiency have hypertension and growth retardation. Male infants have premature sexual development, while female infants develop virilization. In 17-hydroxylase deficiency, males develop sexual ambiguity, while females have sexual infantilism.
Complications
Alkalosis may lead to tetany, seizures, and decreased mental status. Metabolic alkalosis also decreases coronary blood flow and predisposes persons to refractory arrhythmias. Metabolic alkalosis causes hypoventilation, which may cause hypoxemia, especially in patients with poor respiratory reserve, and it may impair weaning from mechanical ventilation. By increasing ammonia production, it can precipitate hepatic encephalopathy in susceptible individuals.
Diagnostic Considerations
Severe metabolic alkalosis is a life-threatening condition; recognizing and treating the condition appropriately is important. The diagnosis of metabolic alkalosis is difficult to miss in patients in the intensive care unit (ICU) because arterial blood gases (ABGs) are measured routinely in most of these patients.
In non-ICU patients, metabolic alkalosis is suspected if electrolytes show an elevated carbon dioxide level. An elevated carbon dioxide level may also be secondary to respiratory acidosis. Because treatments for the 2 conditions differ, differentiating between them by reviewing the clinical condition of the patient and performing ABGs if the elevation in carbon dioxide is severe is important. In addition, check serum K+ and ionized Ca2+ because metabolic alkalosis is often associated with hypokalemia and decreased serum ionized Ca2+ levels.
For a discussion of metabolic alkalosis in children, see Pediatric Metabolic Alkalosis. For a general review of acid-base regulation, see Metabolic Acidosis.
Differential Diagnoses
Approach Considerations
Metabolic alkalosis is diagnosed by measuring serum electrolytes and arterial blood gases. If the etiology of metabolic alkalosis is not clear from the clinical history and physical examination, including drug use and the presence of hypertension, then a urine chloride ion concentration can be obtained. Metabolic alkalosis secondary to volume depletion is usually associated with a low urine chloride ion concentration (< 20 mEq/L).
For an algorithmic approach metabolic alkalosis, see the image below.*
[*And for comprehensive information on each of the diagnoses in the flow chart, please type in the diagnosis in the search box on emedicine.medscape.com]
Serum Anion Gap
Calculation of the serum anion gap may help to differentiate between primary metabolic alkalosis and metabolic compensation for respiratory acidosis. The anion gap is frequently elevated to a modest degree in metabolic alkalosis because of the increase in the negative charge of albumin and the enhanced production of lactate.
Normal values for the anion gap vary in different laboratories and between individual patients, however, so it is important to know the range of normal for the particular clinical laboratory and the prevailing baseline value for a particular patient. [9] In any event, the only definitive way to diagnose metabolic alkalosis is with a simultaneous blood gases analysis that shows elevation of both pH and PaCO2 and increased calculated bicarbonate.
Anion Gap Reference Range
The anion gap is the difference between primary measured cations (sodium Na+ and potassium K+) and the primary measured anions (chloride Cl– and bicarbonate HCO3–) in serum. This test is most commonly performed in patients who present with altered mental status, unknown exposures, acute renal failure, and acute illnesses. [1] See the Anion Gap calculator.
The reference range for the anion gap is as follows: [2]
[Urine Anion Gap Reference Range]
For the urine anion gap, the most prominently unmeasured anion is ammonia. Healthy subjects typically have a gap of 0 to slightly normal (< 10 mEq/L). A urine anion gap of more than 20 mEq/L is seen in metabolic acidosis when the kidneys are unable to excrete ammonia (such as in renal tubular acidosis). If the urine anion gap is zero or negative but the serum AG is positive, the source is most likely gastrointestinal (diarrhea or vomiting). [3]
See Urinary Anion Gap by Dr Chris Nickson, last update
Here are excerpts from the above:
OVERVIEW
Urinary Anion Gap = [Na+]+ [K+] – [Cl–]
- the cations normally present in urine are Na+, K+, NH4+, Ca2+,and Mg2+.
- the anions normally present are Cl–, HCO3–, sulphate, phosphate and some organic anions.
- only Na+, K+ and Cl– are commonly measured in urine so the other charged species are the unmeasured anions (UA) and cations (UC).
CLINICAL USE
- the urinary anion gap can help to differentiate between GIT and renal causes of a hyperchloraemic metabolic acidosis.
- it has been found experimentally that the Urinary Anion Gap (UAG) provides a rough index of urinary ammonium excretion.
- ammonium is positively charged so a rise in its urinary concentration (ie increased unmeasured cations) will cause a fall in UAG
[See the post for excellent brief discussion of pathophysiology]
SUMMARY
[See Urine Anion Gap Reference Range above]
- low urinary AG = GI loss of base
- no change in urinary AG = renal loss of base
- negative urinary AG = severe diarrhea
- positive urinary AG = altered urinary acidification
Urine Anion Gap From MDCalc
Detects urine acidosis for evaluation of non-gap metabolic acidosis.When To Use
- The patient with an unexplained non-gap metabolic acidosis.
- The patient with a mixed metabolic acidosis.
- Evaluation for suspected RTA.
Why Use This simple urine test can help differentiate the causes of a non-gap metabolic acidosis (HARDUP):
- Hyperchloremia
- Acetazolamide, Addison’s disease
- Renal tubular acidosis
- Diarrhea, vomiting, ileostomies, fistulae
- Ureteroenteric fistulae
- Pancreatoduodenal fistulae
MANAGEMENT
Remember that hyperchloremic fluids can worsen these acidoses; consider fluids with lower chloride concentrations (like LR).
Metabolic Alkalosis Workup from emedicine.medscape.com
Updated: Nov 01, 2018
- Author: Christie P Thomas, MBBS, FRCP, FASN, FAHA
For details of the metabolic alkalosis workup please see the following sections from the above link:
- Urine Sodium Ion Concentration
- Plasma Renin Activity and Aldosterone level
- Evaluations for Primary Hyperaldosteronism, Cushing Syndrome, and Apparent Mineralocorticoid Excess
- Evaluation for Congenital Adrenal Hyperplasia Variants
- Diuretic Screen, Adrenal Imaging, and Renovascular Hypertension Imaging
- Gene Analysis
Metabolic Alkalosis Treatment & Management From emedicine.medscape.com
Updated: Nov 01, 2018
- Author: Christie P Thomas, MBBS, FRCP, FASN, FAHA
Chloride-Responsive Alkalosis
Chloride-Resistant Metabolic Alkalosis
Specialized Therapies in All Types of Metabolic Alkalosis
Consultations
Medication Summary
Resources:
(1) Metabolic Alkalosis
Updated: Nov 01, 2018 from emedicine.medscape.com
Author: Christie P Thomas, MBBS, FRCP, FASN, FAHA