Today, I review, link to, and excerpt from Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Riann Jenay Egusquiza 1 2, Bruce Blumberg 1 2 3. Endocrinology. 2020 Mar 1;161(3):bqaa024. doi: 10.1210/endocr/bqaa024.
There are 108 similar articles in PubMed.
The above article has been cited by 38 articles in PubMed.
All that follows is from the above resource.
Abstract
The incidence of obesity has reached an all-time high, and this increase is observed worldwide. There is a growing need to understand all the factors that contribute to obesity to effectively treat and prevent it and associated comorbidities. The obesogen hypothesis proposes that there are chemicals in our environment termed obesogens that can affect individual susceptibility to obesity and thus help explain the recent large increases in obesity. This review discusses current advances in our understanding of how obesogens act to affect health and obesity susceptibility. Newly discovered obesogens and potential obesogens are discussed, together with future directions for research that may help to reduce the impact of these pervasive chemicals.
Keywords: EDCs; adipogenesis; endocrine disrupting chemicals; obesity; obesogen; transgenerational.
© Endocrine Society 2020.
Obesity is a pandemic that has reached worldwide proportions, affecting essentially every country (1). The most dramatic increase in obesity incidence has occurred over the past 5 decades. Approximately 39.6% of US adults were characterized as obese in 2016, compared to 13.4% in 1980 (2). Even more alarming, the incidence of obesity in children has also been increasing, with 18.5% of US children being characterized as obese in 2016, compared to just 4% before 1980 (2). In some countries, the prevalence of childhood obesity exceeds that of adults (3). Obesity and high body mass index (BMI) are not just cosmetic concerns, but are also associated with comorbidities such as increased risk for heart disease, type 2 diabetes and other metabolic diseases, and cancers, and have contributed to approximately 4 million deaths worldwide between 1980 and 2015 (3). There is an urgent need to understand all the factors contributing to obesity to best implement effective prevention and treatment approaches that have so far proved elusive.
The Obesogen Hypothesis
The predominant medical explanation continues to be that obesity is the result of a simple imbalance between excessive calorie intake and insufficient energy expenditure—the energy balance or “calories in, calories out” model. However, recent studies have demonstrated that this simple paradigm cannot explain the increase in BMI seen in recent years. A study analyzing National Health and Nutrition Examination Survey (NHANES) data compared BMI between US adults in 1988 and 2006 and found a 2.3-kg/m2 increase in adult BMI in 2006 compared with 1988, even at the same amount of caloric intake and energy expenditure (4). Moreover, the quality of carbohydrate calories consumed (high vs low glycemic load) appears to be more important than the total quantity of calories consumed (5, 6). Genetics is widely believed to be associated with obesity; however, known gene variants can explain only 2.7% of the individual variation in BMI (7). Therefore, the 2 most commonly given explanations—genetics and energy balance—cannot fully explain the substantial increases in obesity incidence observed worldwide.
Multiple environmental factors can affect obesity susceptibility (reviewed in 8, 9). These include the gut microbiome composition (10, 11), stress (12), and disrupted circadian rhythms (13), to name a few. Environmental stressors experienced during fetal development have significant impacts on obesity susceptibility later in life. For example, mothers who were in their first and second trimester of pregnancy during the Dutch Hunger Winter of 1944 to 1945 gave birth to children who were predisposed to obesity later in life compared to mothers not exposed to famine during pregnancy (14). Maternal smoking during pregnancy was also shown to lead to a predisposition toward obesity later in life for prenatally exposed children (reviewed in 15).
In 2003, Jerry Heindel put endocrine-disrupting chemicals (EDCs) and obesity on the same map for the first time (16). His idea followed from a proposal originally made by Paula Baillie-Hamilton that increased chemical usage since World War II was responsible for the rapid increase in obesity over the same time period (17). Although it was not justifiable to link increased chemical use, per se, to obesity, Heindel’s proposal that EDCs might be influencing obesity was reasonable because nearly every aspect of the control of appetite, satiety, metabolism, and fat storage is regulated by the endocrine system. Heindel’s proposal eventually ignited research in the area of obesity among researchers already working on EDCs (reviewed in 18*).
*History of the Obesogen Field: Looking Back to Look Forward [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Jerrold J. Heindel.
[The above article] is part of the Research Topic: Endocrine Disruptors and Metabolism. View all 22 articles.
Resuming excerpts from Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals
The idea of EDCs as factors in obesity did not crystallize until it was recognized that certain EDCs could activate nuclear hormone receptors important for the development of white adipocytes, such as peroxisome proliferator–activated receptor γ (PPARγ) (19). Further support came from the findings that EDCs such as tributyltin (TBT) could lead to increased adipogenesis in cell culture models (20-22), bind to and activate PPARγ and its heterodimeric partner, the 9-cis retinoid X receptor (RXR), in vitro (21, 22), and lead to increased adiposity in vivo (22). The identification of chemicals that activated PPARγ to promote adipocyte differentiation and white adipose tissue (WAT) accumulation led to the coining of the term obesogen (23).
Obesogens were defined functionally as chemicals that lead to increased WAT accumulation, in vivo, after exposure. The environmental obesogen hypothesis holds that obesogen exposure is an under-recognized and understudied factor in the obesity pandemic (reviewed in 23, 24). Although the obesogen hypothesis was initially controversial, many chemicals known to be obesogenic in animal models are also associated with increased obesity prevalence, BMI, and body weight in humans (9). Research in this area has burgeoned and numerous recent reviews have summarized aspects of obesogen research (eg, 8, 18, 25, 26). It was subsequently recognized that obesogens may have more diverse effects on metabolism than just contributing to obesity; although, obesity may be a key contributor to such effects. These include type 2 diabetes, nonalcoholic fatty liver disease, and the central control of metabolism. Thus, it can be argued that most obesogens are a subset of a larger class of chemicals termed metabolism-disrupting chemicals*, not all of which are obesogens (reviewed in 9, 27). Here we summarize what is known about the mechanisms underlying obesogen action, discuss newly identified obesogens and potentially obesogenic chemicals and propose important areas for future research.
Link to the results of a Google search for metabolism disrpting chemicals.
Classic Effects and Mechanisms of Obesogens
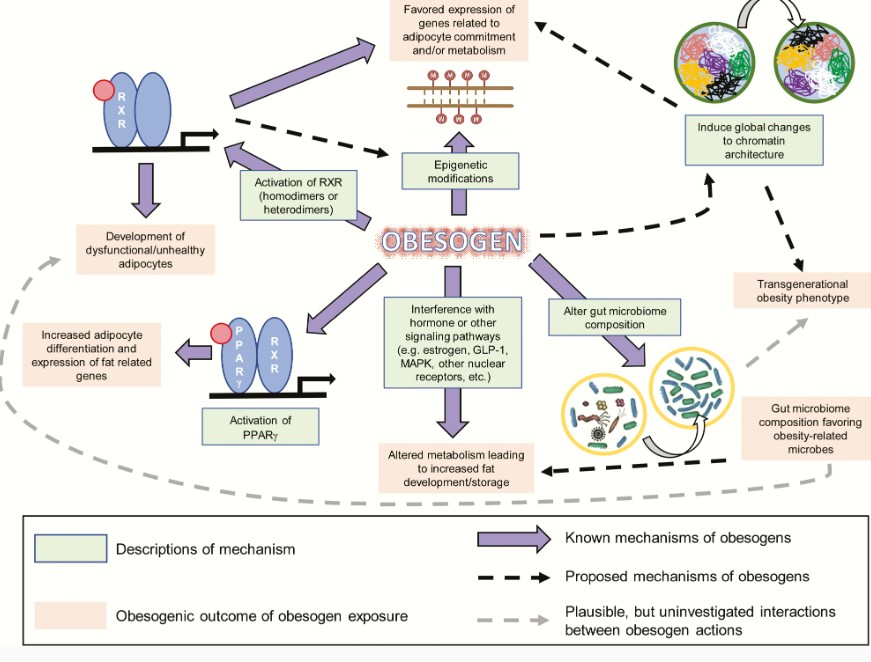
Obesogens affect the differentiation of white adipocytes, in vitro, and the storage of fat, in vivo, in multiple model organisms (reviewed in 8, 9)(Fig. 1). We distinguish bona fide obesogens that induce increased WAT weight, in vivo, from potential obesogens that can induce adipogenesis, in vitro, but have not yet been demonstrated to induce WAT accumulation in vivo. Numerous potentially obesogenic compounds have been identified using in vitro assays that assess the ability of candidate chemicals to promote differentiation of established cell lines such as 3T3-L1 preadipocytes (28, 29) or primary mouse and human multipotent mesenchymal stromal stem cells (also known as mesenchymal stem cells or MSCs) into mature adipocytes (29, 30). Many chemicals that promote differentiation of white adipocytes in these assays activate PPARγ and/or RXR (29, 30). This is not surprising because the PPARγ:RXR heterodimer is considered to be the “master regulator of adipogenesis” (31).
Diagram of known and proposed mechanisms and effectsof obesogen exposure. Known mechanisms through which obesogens act are demonstrated by solid purple arrows. Proposed mechanisms are shown by arrows with a dashed black line. Plausible, but uninvestigated, interactions between obesogen actions are shown by arrows with a dashed gray line. Known mechanisms are described in green boxes, and the outcomes of obesogen exposures are shown in pale red boxes.
One of the most well-characterized obesogens is the organotin, TBT. Organotins are widely used in industry and to some extent in agriculture. Human exposure to organotins can occur via the diet such as seafood contaminated by TBT used in marine shipping applications (32), or as fungicides for paper mills and industrial water systems (reviewed in 33). Triphenyltin use as a fungicide and miticide on high-value food crops presents more opportunities for human exposure (34). TBT contaminates polyvinyl chloride plastics, and organotins are found in samples of house dust (35, 36).
TBT binds to and activates PPARγ and RXR at environmentally relevant (nanomolar; nM) levels, promoting adipogenesis and lipid accumulation (21, 22, 37). Human and mouse MSCs and 3T3-L1 preadipocytes were induced to differentiate into white adipocytes via a PPARγ-dependent pathway after exposure to nM levels of TBT (38, 39). Prenatal TBT exposure diverted bone marrow–derived MSCs preferentially toward the adipose lineage and away from the bone lineage in exposed mice (39) and in mouse MSCs, in vitro (40).
Epidemiological studies of TBT exposures and effects are scant. A longitudinal Finnish cohort study positively associated placental TBT levels with infant weight gain, an established risk factor for adult obesity (50). A recent analysis of NHANES data revealed a strong link between elevated urinary total tin levels and diabetes (51). Human exposure to tin is ubiquitous (52), and it was just shown that plastic specimen containers strongly bind organotins (particularly TBT), sharply impairing recovery (53). Therefore, previous studies of organotin levels in human specimens (eg, 54) probably substantially underestimated TBT levels because of their use of plastic containers during processing and analysis.
A recent clinical study that strongly supports the obesogen hypothesis found that people with the highest blood levels of perfluorinated chemicals had lower resting metabolic rates and regained weight faster after dieting than those with the lowest levels (55). In agreement with predictions from rodent studies (39, 40), the same group showed that humans with the highest blood levels of perfluoroalkyl compounds had lower bone mineral density at baseline and lost bone mineral density faster in a weight-loss trial (56).
Similar obesogenic effects have been observed with other environmental chemicals, such as phthalates, persistent organic pollutants, and components of plastics and epoxy resins. The phthalate MEHP (mono-2-ethylhexyl phthalate) induced adipogenesis in 3T3-L1 preadipocytes via activation of PPARγ (57). DEHP (di-2-ethylhexyl phthalate) induced expression of adipogenic genes in vivo and an obesity phenotype in mice following perinatal exposure (58). Prenatal exposure to bisphenol A (BPA) has been linked to various adverse health effects including reproductive and behavioral issues (59), fat gain in mice and rats (60, 61) and other metabolic outcomes such as type 2 diabetes (62, 63). Some data support the possibility that BPA influences adipogenesis as a PPARγ agonist, although BPA is a relatively weak activator of PPARγ in vitro (64-66). Others have proposed that BPA may induce its obesogenic effects indirectly via its ability to bind to estrogen receptors and interfere with estrogen signaling (67).
Effects of obesogens acting through other nuclear receptors such as the glucocorticoid receptor, estrogen receptors, and androgen receptors have been reported and discussed in detail elsewhere (reviewed in 9, 26). More recently, it has been observed that chronic 52-week exposure of male mice to a mixture of 6 pesticides commonly used in France, at doses equivalent to the tolerable daily intake of each pesticide, led to increased body weight, WAT weight, and glucose intolerance (68). In contrast, similarly exposed female mice exhibited elevated fasting glucose, increased ratio of reduced to oxidized glutathione in the liver, and perturbed levels of microbiome-related urinary metabolites. Loss of the xenobiotic receptor constitutive androstane receptor (CAR) prevented body weight gain and changes in glucose metabolism in male mice, whereas females exhibited increased toxicity, higher body weights, and elevated mortality rates in the absence of CAR (68).
Many obesogens that have been identified using in vivo studies act through nuclear hormone receptor–dependent mechanisms, as do many of those detected using in vitro adipogenesis assays (reviewed in 69). In addition, interaction between nuclear receptors and cross-talk between signaling pathways has been reported (70). However, some of the effects observed in vitro (29) and in vivo, especially transgenerational effects, (25) have not been linked directly to the activation of particular nuclear hormone receptors. Therefore, the mechanisms through which these compounds act appear to be more complex.
Mechanisms of Obesogen Action: Beyond Peroxisome Proliferator–Activated Receptor γ
Because many known obesogens activate PPARγ and induce adipogenesis, PPARγ activation is widely believed to be a major mechanism through which obesogens can contribute to obesity. PPARγ continues to be the receptor most commonly targeted in screening assays for obesogens (28, 71, 72). However, recent studies have demonstrated alternative and novel mechanisms of obesogen action. These include activation of RXR to induce adipocyte lineage commitment and impair adipocyte health (73, 74), activation of multiple other nuclear receptors, induction of epigenetic modifications in fat tissue (75), alteration of chromatin accessibility or architecture (41, 76), and induction of gut microbiome dysbiosis (77-79). Thus, obesogens have a broad and diverse spectrum of actions. This section discusses some PPARγ-independent mechanisms of obesogens action. A summary of the known and proposed mechanisms through which obesogens can act, together with their possible effects, is illustrated in Fig. 1.
Adipocyte commitment
Adipocyte health
Gut microbiome dysbiosis
Possible Mechanisms Underlying Transgenerational Effects of Obesogen Exposure
An intriguing result is that the effects of early-life obesogen exposure can be transmitted to future generations. When pregnant F0 mouse dams were treated with TBT, F1 animals were exposed as embryos, and F2 were exposed as germ cells within F1. F3 and subsequent generations were not exposed; effects in these generations are considered to be transgenerational and permanent (100, 101). It was shown that the effects of TBT treatment on obesity were transgenerational and could be detected in the F1, F2, F3, and F4 descendants of F0 mice exposed during pregnancy (42) or during pregnancy and lactation (41). Interestingly, transgenerational obesity was not observed in similar experiments using the strong PPARγ activator rosiglitazone; therefore, pathways in addition to PPARγ may be required to produce the transgenerational phenotype (42). Alternatively, it might be possible that the “unhealthy” adipocytes produced by TBT exposure are responsible for the transgenerational phenotypes in adipose tissue; this is a ripe area for future study. It was proposed that obesogen exposure can permanently reprogram MSCs to favor the adipose lineage (39). Gene expression in MSCs taken from F1 to F3 generation mice after F0 exposure throughout pregnancy was also biased toward the adipogenic lineage (42). It was suggested that TBT exposure promoted epigenomic changes favoring the development of obesity (41); thus, this may be an example of a maternal programming event leading to a life-long phenotype.
In addition to TBT, heritable effects of several environmental chemicals on obesity have been demonstrated, albeit at relatively high doses. Plastic components such as BPA, diethylhexyl and dibutyl phthalates (58), the pesticide methoxychlor (102), a mixed hydrocarbon mixture (jet fuel JP-8) (103), and the once widely used pesticide, DDT (104) all induced transgenerational obesity in rats, observed in the F3 and/or F4 offspring of following ancestral prenatal or perinatal obesogen exposure to the F0 dams (58, 101-104). The mechanisms underlying these transgenerational effects remain unclear. Some proposed mechanisms for transgenerational effects of obesogen exposure are discussed in this section and illustrated in Fig. 1. Transgenerational effects of obesogen exposure are particularly concerning because current risk assessment paradigms do not consider this “generational toxicology” (105).
Epigenetic modifications
Start here.
Chromatin accessibility
Newly Discovered Obesogens (New Threats)
Dibutyltin
Bisphenol A analogues
Acrylamide
Surfactants
Food additives
Pesticides
Future Directions
Although the environmental obesogen field is just 15 years old, it is becoming clear that chemical exposures may be important contributors to the obesity pandemic. Many advances have been made into potential mechanisms underlying obesogen action and how obesogen exposure may predispose humans and animals to obesity (Fig. 1). However, we have only just scratched the surface and need to learn much more about the number of obesogens that exist, how they act, and how we can best protect ourselves and future generations from their harmful impacts. A combination of mechanistic studies in cell and animal models together with longitudinal epidemiological and biomonitoring studies in humans will be required for a full assessment of the risks and costs of these exposures to public health. Early estimates suggest that these costs may be substantial (150, 151).