Today, I review, link to, and embed, Echocardiographic evaluation of diastolic function in the setting of pulmonary hypertension. Vineet Agrawal 1, Benjamin F Byrd 3rd 1, Evan L Brittain 1. Pulm Circ. 2019 Feb 5;9(1):2045894019826043. doi: 10.1177/2045894019826043. eCollection 2019 Jan-Mar. [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
All that follows is from the above resource.
Abstract
Heart failure due to diastolic dysfunction and pulmonary hypertension are frequent comorbid conditions with significant morbidity and mortality. Identifying the presence and etiology of diastolic dysfunction in the setting of pulmonary hypertension remains challenging despite profound therapeutic and prognostic implications. Additionally, there is little guidance in identifying and parsing etiology of diastolic dysfunction in patients found to have pulmonary hypertension. This review discusses the complex interplay between left ventricular diastolic dysfunction and pulmonary hypertension. With an explicit focus on the use of echocardiography for determination of diastolic dysfunction and etiology of pulmonary hypertension, this review also provides a comprehensive review of the literature and provides a framework by which to assess diastolic dysfunction echocardiographically in the setting of pulmonary hypertension.
Keywords: pulmonary hypertension, diastolic dysfunction, echocradiography
Introduction
With a growing incidence and prevalence worldwide, heart failure remains a condition with limited treatment options and poor prognosis.1,2 Left ventricular diastolic dysfunction, defined by impaired relaxation of the myocardium, is a hallmark of heart failure in patients who present with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF).3 The presence of even asymptomatic diastolic dysfunction portends a worse prognosis for patients regardless of the underlying etiology.4–6 Pulmonary hypertension frequently coexists with left heart diastolic dysfunction.7–10 Since both pulmonary hypertension and diastolic dysfunction can be a primary cause of symptoms such as exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and exercise intolerance, distinguishing the primary cause of symptoms and pathology carries significant prognostic and therapeutic implications.
A key determinant in the primary cause of symptoms in patients with both diastolic dysfunction and pulmonary hypertension is the evaluation of left atrial filling pressures and left ventricular end-diastolic pressures (collectively referred to as left sided filling pressures).8,11–13 Invasive cardiac catheterization with or without provocation by exercise or fluid challenge is the gold standard for measuring left-sided filling pressures; however, it can be associated with misclassification of patients with concomitant pulmonary hypertension due to technical/interpretative errors or lack of evidence of elevated filling pressures in the absence of provocative maneuvers.5,12,14,15 Non-invasive assessment of left sided filling pressures allows for a more global structural assessment of the heart to provide additional clues that may aid in the differentiation of the primary cause of symptoms. In addition, non-invasive approaches eliminate periprocedural risk, potentially reducing health care costs and allowing for a potential method for serial monitoring of response to therapies. While recent guidelines have created a more simplified and unified approach to the non-invasive assessment of diastolic function in echocardiography with a focus on a disease-oriented approach, there is limited guidance on and data supporting the accurate assessment of diastolic function in pulmonary hypertension.3
Complex interplay between left ventricular diastolic function and pulmonary vascular function
While many previous studies have focused on the complex coupling between the right ventricle and pulmonary artery that is well studied in the setting of pulmonary hypertension,16,17 there also exists a complex interaction between the left ventricle and pulmonary circulation.
A number of studies have reviewed the role of left ventricular impaired relaxation resulting in passive congestion and chronic pulmonary vascular changes.18,19 Other studies have also shown that the chronic pulmonary vascular changes, whether primary or secondary, can themselves impair diastolic relaxation of the myocardium globally.8,18,20–22 Additionally, the presence of pulmonary hypertension is associated with progressive structural changes on both the right and left side of the heart.23,24
Pulmonary hypertension as a consequence of left ventricular diastolic dysfunction
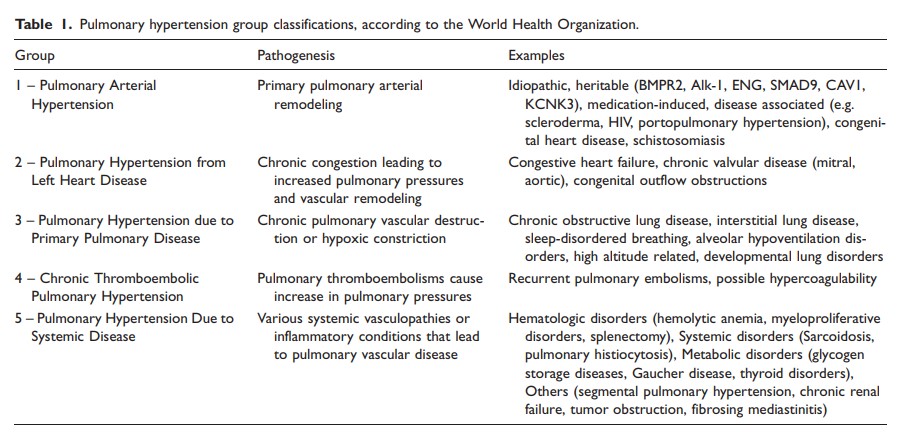
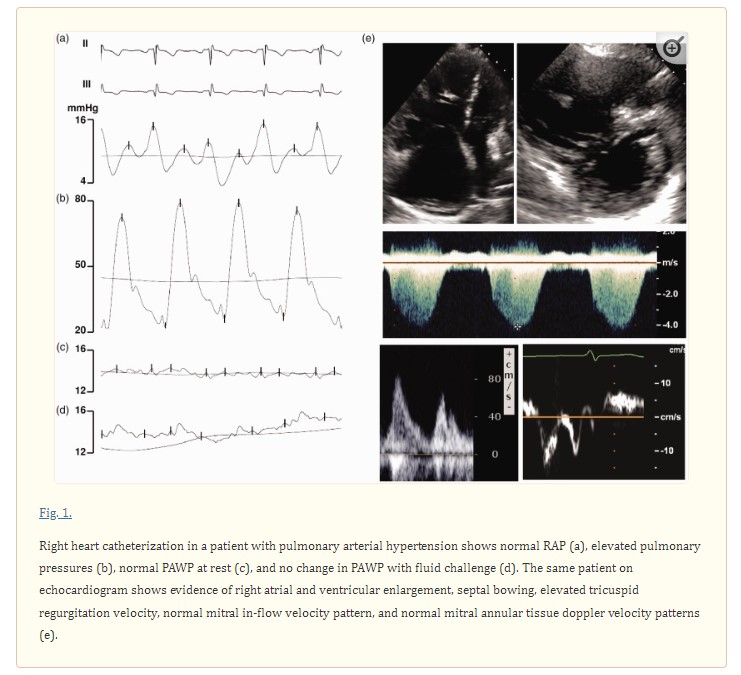
The most commonly understood mechanism by which left ventricular diastolic dysfunction and pulmonary hypertension coexist is in the setting of pulmonary hypertension secondary to left sided heart disease. Pulmonary hypertension is classically divided into five groups by the World Health Organization (WHO) (Table 1),25
with group 2 representing patients with pulmonary hypertension due to elevated left heart filling pressures that are thought to be the primary cause of pulmonary hypertension. Defined invasively by a measured mean pulmonary artery pressure (PAP) ≥ 25 mmHg and a mean pulmonary arterial wedge pressure (PAWP) > 15 mmHg at rest, group 2 PH patients are further subdivided into patients with isolated post-capillary pulmonary hypertension (Ipc-PH) and combined pre- and post-capillary pulmonary hypertension (Cpc-PH).26 The latter condition is characterized by the development of pulmonary vascular remodeling leading to increased pulmonary vascular resistance,27 Group 2 PH is the most prevalent form of pulmonary hypertension in developed countries and is rapidly becoming the most prevalent form of pulmonary hypertension worldwide,11,25,28 and studies utilizing provocative maneuvers such as exercise or fluid challenge with right heart catheterization suggest that the true prevalence of group 2 PH may be underestimated due to misclassification (Figs. 1 and and22).11,12,29 A number of studies have now evaluated the prevalence of occult post-capillary pulmonary hypertension in patients who are initially diagnosed with pulmonary arterial hypertension (PAH). In one study of 287 patients who all underwent fluid challenge for evaluation of pulmonary hypertension, 22% of them were found to have elevated PAWP ≥ 15 mmHg after fluid challenge.11 In another cohort of 53 patients with scleroderma-associated pulmonary hypertension, 45% of patients had evidence of occult post-capillary pulmonary hypertension after fluid challenge.30 However, when using a stricter threshold for an abnormal fluid challenge, D’Alto et al. found, in their cohort of 190 patients, that about 7% of patients would be reclassified as having post-capillary pulmonary hypertension.29 All in all, the presence of patients with occult post-capillary pulmonary hypertension has decreased the accuracy of current estimates of prevalence. Notably, current guidelines do not yet routinely recommend provocative challenge for identifying patients with occult post-capillary pulmonary hypertension.
The prevalence is further affected by the accepted threshold of 25 mmHg. There is substantial data suggesting that patients with “borderline” pulmonary hypertension have a higher risk of mortality and hospitalization.31–35 Many of these patients have increased left sided filling pressures by cardiac catheterization,32 and left atrial and ventricular structural changes,11 suggesting left ventricular diastolic dysfunction as the driver of pathology.
Impaired left ventricular diastolic filling as a result of PAH
It has been noted that a subset of patients who are diagnosed with PAH also exhibit left ventricular diastolic dysfunction. Two possible mechanisms have been proposed to contribute to this pathogenesis.
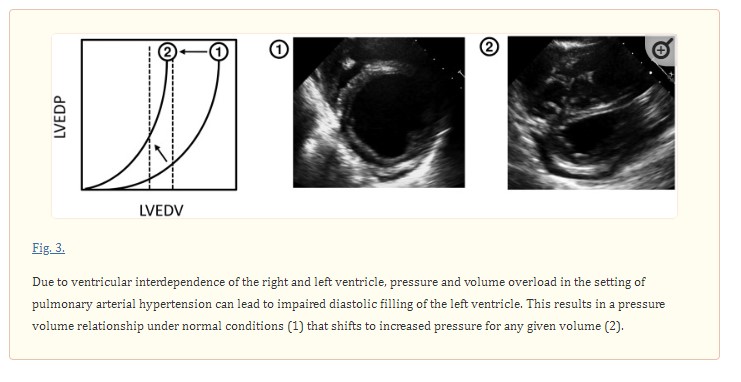
One mechanism that has been proposed is impaired diastolic filling of the left ventricle as a consequence of significant pre-capillary pulmonary hypertension. This occurs due to ventricular interdependence, a phenomenon in which left and right ventricular volumes/pressures are interdependent due to the presence of an interventricular septum and pericardial sac that limits volume expansion of the heart.20–22,36–38 Under normal conditions, this interdependence is minimal and without clinical consequences, but significant pulmonary hypertension can significantly impair left ventricular diastolic filling by significantly altering the geometry of the left ventricle due to septal bowing from a pressure and volume overloaded right ventricle (Fig. 3). Notably, impaired ventricular filling as a result of enhanced ventricular interdependence is functionally distinct from intrinsic impaired myocardial relaxation. In addition, prior studies investigating this method have shown up to a 3 mmHg increase in PAWP.14,29,39,40 Thus, while it is important to consider these changes in filling pressures when determining the etiology of pulmonary hypertension, ventricular interdependence may not be the sole cause of significant left ventricular diastolic dysfunction in patients with PAH.
The second mechanism that has been proposed in various studies is common genetic pathogenesis that gives rise to both pulmonary vascular remodeling and cardiomyopathy. This is supported by a number of studies that have found contractile deficits in isolated myocytes from the left ventricle and septum of patients with idiopathic PAH (IPAH) as well as systemic sclerosis-associated PAH.41,42 In addition, in a cohort of patients with IPAH, heritable PAH, connective tissue disease PAH, and chronic thromboembolic disease-related PAH, non-invasive assessment of LV diastolic function by cardiac magnetic resonance imaging (cMRI) suggested global impairment in LV diastolic function in patients with PAH compared with controls.43 While it is possible that diastolic dysfunction is an acquired change in the LV of patients with PAH, LV diastolic impairment has also been identified in children with PAH by echo.44 While cardiac manifestations of PAH have been studied in patients in certain forms of PAH,37 the overlap between diastolic cardiomyopathy and PAH remains incompletely understood.
Non-invasive evaluation of diastolic function by echocardiography
Non-invasive evaluation of diastolic function is possible through multiple imaging modalities.45 In particular, cardiac MRI is increasingly being utilized for assessment of cardiac structure and function. This has been discussed extensively in a number of recent review articles,46,47 although not studied specifically in the setting of pulmonary hypertension. The present review focuses on echocardiography as it is currently the most commonly used method for assessing diastolic function non-invasively.
Recommendations for echocardiographic determination of diastolic function have recently been updated, with increased emphasis on high specificity of diagnosis and reproducibility of measures.3
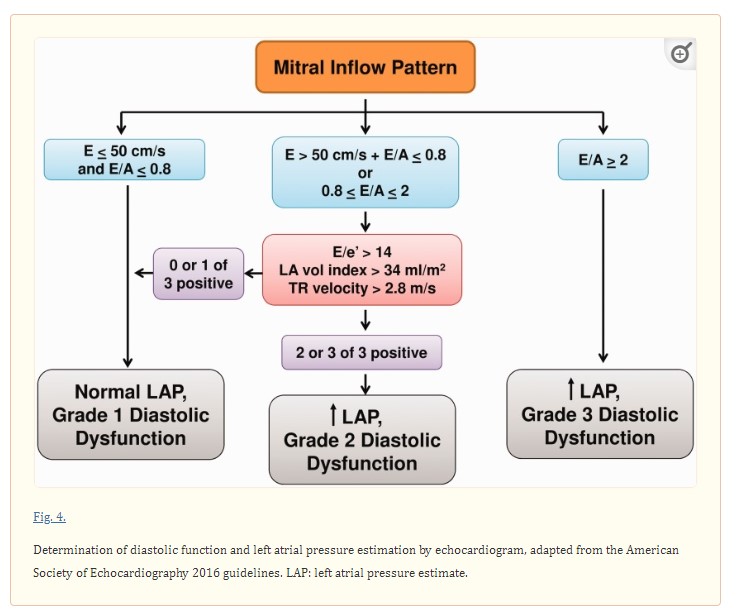
The four recommended variables that are reproducibly associated with diastolic dysfunction are the mitral annular tissue Doppler velocity e’ (septal e’ < 7 cm/sec and/or lateral e’ < 10 cm/sec), left atrial maximal volume index (> 34 ml/m2), average mitral in-flow velocity to annular tissue doppler velocity ratio (E/e’ > 14), and peak tricuspid regurgitant velocity (>2.8 m/s).
Notably, the recommended cutoffs are based on prior studies showing high specificity for diastolic dysfunction.48 Further characterization of the severity, or grade, of diastolic dysfunction is then based on the number of abnormal parameters as well as the E velocity and E/A ratio (Fig. 4).
The applicability, accuracy, and performance of these and prior non-invasive algorithms for estimation of diastolic function has been a point of significant controversy in the literature. Studies have highlighted the cutoffs for variables as misclassifying both unaffected and affected individuals, and they have pointed out the pitfalls in a static cutoff that does not account for dynamic normal changes that occur with aging.49 Epidemiologic studies have also highlighted the significant differences in prevalence of diastolic dysfunction various population cohorts between prior and current guidelines (Table 2), with variation in the prevalence of diastolic dysfunction up to 30% between different guidelines and a significant proportion still being labeled as “indeterminant” based on guidelines.50,51 The ability of the guidelines to accurately distinguish normal vs abnormal left sided filling pressures has shown mixed results. While some studies have suggested high degree of correlation between non-invasive and invasive measures of left sided filling pressures in patients,13,52–54 other studies have suggested a modest correlation at best.55–58 A key difference may be that the studies that were enriched for patients with a known predisposition to abnormal left sided filling pressures, i.e. heart failure, showed poorer performance of non-invasive correlates. Of particular note is a systematic review of patients from 9 studies of patients with diastolic heart failure to compare invasive hemodynamics with echocardiographic parameters, and 18 studies to compare echocardiographic parameters to clinical outcomes.55 In aggregate, the systematic review found only modest evidence to support the use of current guidelines in the assessment of elevated left sided filling pressures using average E/e’ with a pooled correlation coefficient of 0.56 in all 9 studies. There was additionally a modest association between E/e’ and major adverse cardiac events or heart failure hospitalization (pooled hazard ratio of 1.05 per unit increase in E/e’). This would suggest that further refinement of the thresholds of diastolic function variables may be necessary to more accurately identify abnormal left sided filling pressures in patients with a higher pre-test probability for abnormal left sided filling pressures, i.e. patients who suffer from heart failure or post-capillary pulmonary hypertension.
Pitfalls in diastolic function assessment: tricuspid regurgitation velocity
Although used in current algorithms to identify the presence or absence of diastolic dysfunction, tricuspid regurgitant velocity, when detectable, is elevated in all etiologies of pulmonary hypertension, thus limiting its specificity.8,18,20–22,35 The classic approach to non-invasive estimation of pulmonary pressures is the use of a tricuspid regurgitant velocity using Bernoulli’s equation. When added to an estimation of the right atrial pressure by assessment of the inferior vena cava (IVC), this pressure reasonably approximates invasively measured pulmonary artery systolic pressure.59,60 When simultaneously measured and interpreted by readers with high inter-reader reproducibility, the correlation between TR velocity derived and invasively measured PA systolic pressures is high.59,61,62 However, in clinical populations, this correlation declines to 0.6–0.8.63–65 This is thought to be due to a number of pitfalls in the use of tricuspid regurgitant velocity as the sole determinant of pulmonary artery pressures. In conditions of a compensated right ventricle in the setting of high pulmonary and right ventricular pressures, and in conditions of equalization of pressures between the right atrium and right ventricle, tricuspid regurgitant velocity may be artificially low, and may underestimate the degree of pulmonary hypertension. Additionally, the estimation of pulmonary pressures is dependent on accurate assessment of the right atrial pressure by IVC assessment, which has diagnostic limitations. The presence of any primary right sided valvular disease can further affect accurate assessment of pulmonary pressures by this method. Finally, the TR velocity envelope is often incomplete or “cut-off” in severe TR, leading to underestimation of PA systolic pressure.63,65–68
Pitfalls in diastolic function assessment: left atrial pressure estimation in pulmonary hypertension
See article for this section
Refined algorithms for distinction of pre- and post-capillary pulmonary hypertension
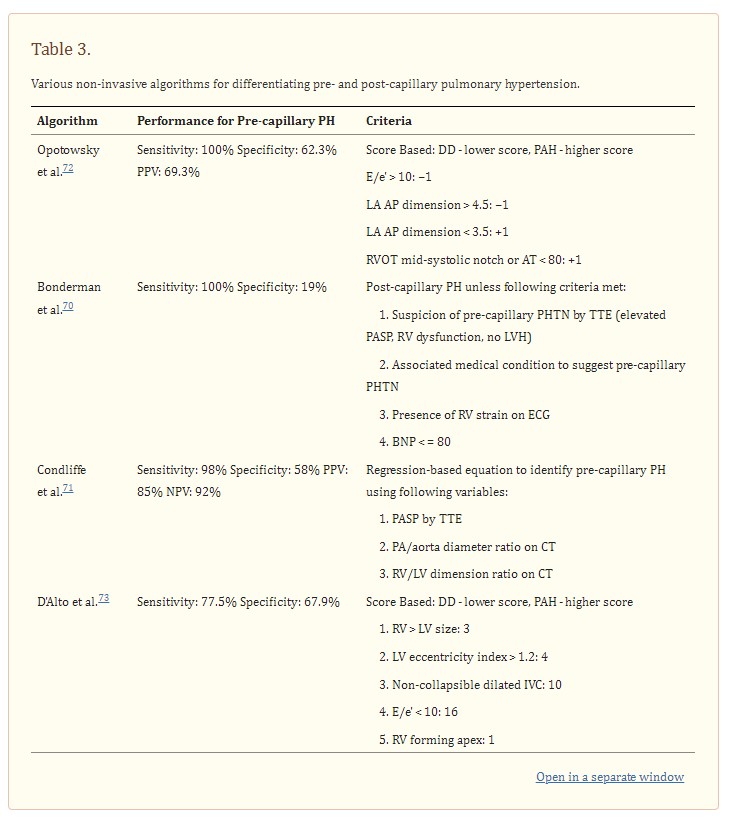
The greatest sensitivity and specificity for detection of left ventricular diastolic dysfunction in the setting of pulmonary hypertension have been reported using multi-variate and multi-modality algorithms (Table 3).
See this section for discussion of the details of the algorithms below.
Emerging modalities for assessment of diastolic function: strain imaging and diastolic stress testing
Strain imaging is increasingly used for assessment of subclinical cardiac dysfunction in various patient populations.74–76 There has also been an emerging role for strain imaging in quantifying and identifying regions of diastolic function in the myocardium.77 Measure of strain by ultrasonography is thought to be a surrogate measure of deformation, and strain rate is a surrogate measure of rate of deformation. In a study by Kasner et al. of 33 patients who underwent strain imaging echocardiography and invasive catheterization for pressure-volume loop analysis simultaneously, LV global strain rate was reduced in patients with diastolic dysfunction compared with controls. Additionally, LV global strain rate during early diastole and isovolumic relaxation correlated with invasively measured left ventricular relaxation constant τ, LV end diastolic pressure, and LV stiffness constant β.77 Strain imaging in pulmonary hypertension has been used to study the mechanics of the right ventricle and response to therapy,78–80 but this technology has not been used to study the left ventricle or distinguish pre- vs post-capillary pulmonary hypertension in the literature.
Similarly, strain imaging has been utilized to study atrial mechanics as a method by which to identify patients with abnormal cardiovascular hemodynamics. In a patient study of 69 patients, which included both patients in sinus rhythm and with atrial fibrillation, speckle tracking echocardiography (STE) was utilized to evaluate contractile function of the left atrium and compared with invasive left and right heart catheterization-derived measurements of intracardiac pressures.81 While no differences were noted in patients with atrial fibrillation, atrial peak positive strain of less than 16% in patients in sinus rhythm was significantly predictive of a PAWP ≥ 18 mmHg, and more predictive than non-invasive estimates such as E/e’. This has further been studied in 49 patients, 45 of whom had pulmonary hypertension (22 of them with pulmonary arterial hypertension, 19 with left sided heart disease as the cause of pulmonary hypertension, 1 with chronic thromboembolic pulmonary hypertension).82 Left atrial strain rate was used to derive a non-invasive estimate of PAWP, and the resulting PVR correlated strongly with catheter-derived measurements (sensitivity 85%, specificity 74%), suggesting that left atrial strain can be a useful measure of left sided filling pressures and pulmonary vascular resistance. While very promising, current limitations of this technology remain the lack of uniform algorithms for measuring strain as well as a lack of consensus on various cutoffs for determining normal vs abnormal strain.
Based on previous studies showing that provocative maneuvers such as fluid challenge or exercise can identify patients with occult post-capillary pulmonary hypertension that otherwise would have been classified as having pulmonary arterial hypertension,11,12 non-invasive stress testing for evaluation of diastolic function has previously been studied in the setting of pulmonary hypertension.7 Studies have shown that stress, via modalities such as exercise, can elicit changes in estimated pulmonary artery pressures,83 tricuspid regurgitation, right and left ventricular functional changes,84 and changes in Doppler based estimates of left ventricular filling pressures.85 However, the application of this approach to the distinction of pre- and post-capillary pulmonary hypertension remains an area of investigation. Limitations of this technology remain technical challenges in obtaining accurate data and feasibility of upscaling this technology to serve as a broad screening tool for all patients with pulmonary hypertension.
Conclusions
Distinction of pulmonary hypertension due to left ventricular diastolic dysfunction and pulmonary arterial hypertension has both prognostic and therapeutic implications. Non-invasive methods to differentiate these sub populations offers the opportunity for better screening and monitoring of therapeutic efficacy without the risks of invasive procedures. Despite multiple non-invasive algorithms proposed for identification for distinguish pre- and post-capillary pulmonary hypertension, future studies are necessary to evaluate these algorithms in the growing subset of patients who have evidence of diastolic dysfunction only after provocation. With the growing prevalence of pulmonary hypertension worldwide and the increasing therapeutic implications of identification of the subtype of pulmonary hypertension in patients, reliable non-invasive identification of diastolic dysfunction in pulmonary hypertension may have profound impact on diagnosis and management.