In addition to today’s resource, be sure and review:
- Clinical applications of the venous excess ultrasound (VExUS) score: conceptual review and case series. Philippe Rola, Francisco Miralles-Aguiar, Eduardo Argaiz, William Beaubien-Souligny, Korbin Haycock, Timur Karimov, Vi Am Dinh & Rory Spiegel. Ultrasound J. 2021 Jun 19;13(1):32. doi: 10.1186/s13089-021-00232-8. [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
- Link To POCUS Assessment Of Venous Congestion, Grand Rounds By Dr. Philippe Rola
Posted on September 23, 2021 by Tom Wade MD
Today, I review, link to and excerpt from EMCrit‘s “EMCrit 367 – Panel: 4 Quadrant Hemodynamic Ultrasound Integration, IVC Ultrasound and Much More!“*
*Scott Weingart, MD FCCM. EMCrit 367 – Panel: 4 Quadrant Hemodynamic Ultrasound Integration, IVC Ultrasound and Much More!. EMCrit Blog. Published on January 27, 2024. Accessed on February 7th 2024. Available at [https://emcrit.org/emcrit/4-quadrant-hemodynamics/ ].
All that follows is from the above outstanding resource.*
*Note to myself: The best way to review Dr. Weingart’s podcast and show notes is to first review each of the three articles below and then after reading each article, review the corresponding part of the podcast and show notes.
Today on the podcast, a bunch of hemodynamics. Originally, this was going to be an interview with Phillipe Rola, friend of the show and Intensivist extraordinaire. Phil surprised me by bringing 3 other friends and authors of the papers discussed:
- Korbin Haycock, MD
- Rory Spiegel, MD
- Jon-Émile Kenny, MD
We primarily discuss the 3 papers below in addition to a ton more on ultrasound and hemodynamics.
All Things IVC
*What every intensivist should know about the IVC
Philippe Rola et al., J Crit Care, https://doi.org/10.1016/j.jcrc.2023.154455.
Although I have excerpted parts of the above article below, it should be reviewed in its entirety.
1. Introduction
With the gradual adoption of Point-of-Care Ultrasound (POCUS) over the last two decades, the inferior vena cava has maintained its place as a controversial tool for the assessment of volume status in the critically ill. Initially prompted by promising studies suggesting a role in assessing volume responsiveness using the superior vena cava, a rash of studies
using respiratory variation were done, with equivocal results and less clinician enthusiasm for its use [1,2]. More recently, the focus has begun to shift towards assessing venous congestion and the important concept of fluid tolerance, prompting a resurgence of interest in the IVC.4. The IVC and Fluid Tolerance
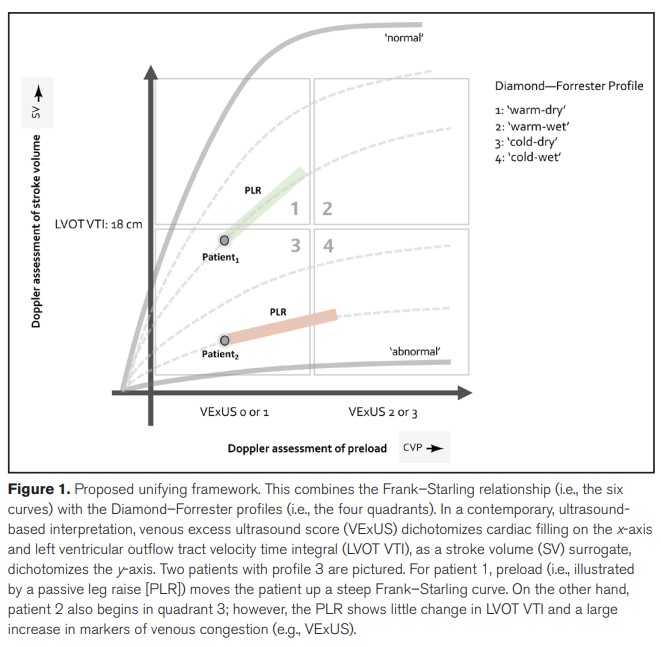
Recently a position statement was put forward on the concept of fluid tolerance, in an attempt to bring attention to what could be a more personalised and physiological approach to resuscitation and deresuscitation [6]. Fluid tolerance refers to the patient’s capacity to receive volume without suffering from its adverse effects. This is a distinct concept and often overlaps with fluid responsiveness rather than intolerance only beginning where responsiveness ends. (Fig. 1).
Hence, given the physiological relationships of the IVC, systemic
congestion begins when venous pressures exceed maximally tolerated tissue afterload. This may also vary with local capillary permeability. While this can happen to inflamed tissue beds with almost any venous pressure, normal tissue will also start to suffer from a decreased effective perfusion as CVP rises beyond a certain point. It should be noted that driving pressure for flow across the capillary is not the MAP minus the
CVP, but rather the precapillary pressure minus the postcapillary pressure. The precapillary pressure is much lower than the MAP as there is a large pressure drop across the precapillary resistors. This makes the reduction to flow contributed by the postcapillary venous pressures
much more important than is generally recognized. Furthermore, because the precapillary arterial resistance is so much higher than the venous resistance, a rise in venous pressure affects intracapillary pressure much more than a rise in arterial pressure. A rise in intracapillary pressure promotes capillary hyperfiltration and the development of interstitial edema–particularly when CVP is high as it is the “afterloading” force that impedes lymphatic return. Vellinga et al. showed
using microcirculatory parameters that a CVP above 12 cm/H2O generally worsened microvascular perfusion [8]. We can thus see how splanchnic congestion begins with distension of the IVC. This was elegantly illustrated with the development of the venous excess ultrasound score (VExUS)* by the group of Beaubien-Souligny, where there is a gradual increase in organ dysfunction when Doppler envelope abnormalities progress beyond the finding of a lone plethoric IVC. In the absence of this plethoric IVC, there seems to be much less risk of congestive dysfunction as measured by acute kidney injury [3]. So, while macrocirculation may improve with fluids, a worsening in microcirculatory parameters indicates a lack of hemodynamic coherence, suggesting an inappropriate therapeutic strategy.
*See and review the two VExUS resources linked to at the start of this post.
Shoc-IV
*SHoC‑IVC: Does assessment of the inferior vena cava by point‑of‑careultrasound independently predict fuid status in spontaneouslybreathing patients with undiferentiated hypotension? CJEM. 2023 Nov;25(11):902-908. doi: 10.1007/s43678-023-00584-1. Epub 2023 Sep 27. Robert Dunfield 1, Peter Ross 1, Daniel Dutton 1, Kavish Chandra 1, David Lewis 1, Frank Scheuermeyer 2, Jacqueline Fraser 3, Patrick Boreskie 4, Chau Pham 4, Sultan Ali 1, Hein Lamprecht 5, Melanie Stander 5, Cameron Keyes 6, Ryan Henneberry 7, Paul Atkinson 8
dilated IVC (>2.5) without resp variation had a good LR + for fluid overload
Discussion
Interpretation of findings
Most importantly, this study confrms that obtaining IVC
measurement by PoCUS is feasible in the ED in spontaneously breathing patients with undiferentiated hypotension. The vast majority of patients (94.1%) had successful (determinate) IVC scans, adequately measuring IVC size and collapsibility. Although patients with a volume overloaded
fuid status were quite infrequent in this patient population,
our analysis indicates that IVC size alone as determined by
PoCUS, along with various combinations of IVC size and
collapsibility could possibly be helpful in identifying these
patients, and potentially avoiding harmful fuid administration. Again, being cautious due to the low number of patients
in question, with subsequently poor confdence intervals,
our data show that a dilated IVC over 2.5 cm in diameter
performs well as a predictor of fuid overload with very high
sensitivity and specifcity; while a lack of IVC collapse is
highly specifc in ruling in the volume overloaded status, and
that combinations of IVC size and collapse can perform well
overall in predicting the volume overloaded fuid status with
dilated IVC and no or minimal collapsibility being the most reliable combinations, with collapsibility in isolation being
21.8 shown to be a somewhat unreliable measure.Clinical implications
The main clinical implication of this study’s fndings is that
PoCUS is a feasible and accurate additional tool to assist in
the initial assessment of fuid status in spontaneously breathing adult patients with undiferentiated hypotension. Such
measurements may be useful in identifying patients who are
fuid overloaded at initial presentation. These patients are
often unstable and require timely care, and therefore having
a test that is rapid and reliable, and helps reduce barriers
to care and could potentially prevent complications from
administering unnecessary and potentially harmful intravenous fuids. Due to the noted study limitations, clinicians
should exercise caution and should incorporate these fndings into their clinical assessment rather than relying on IVC
PoCUS independently for the identifcation of patients with
volume overload.Conclusion
IVC measurement by PoCUS is feasible in spontaneously breathing, hypotensive, adult emergency department
patients, and demonstrates potential value as a predictor of
a volume overloaded fuid status in this population. IVC size
may be the preferred measure.The 4-Quadrant Hemodynamics/Ultrasound Approach
The Paper
unifying_fluid_responsiveness_and_tolerance*
*Unifying Fluid Responsiveness and Tolerance
With Physiology: A Dynamic Interpretation of
the Diamond–Forrester Classification. Crit Care Explor. 2023 Dec 12;5(12):e1022. doi: 10.1097/CCE.0000000000001022. eCollection 2023 Dec. Jon-Émile S Kenny 1 2, Ross Prager 3, Philippe Rola 4, Korbin Haycock 5, John Basmaji 3, Glenn Hernández 6From Mayo Clinic Critical Care Grand Rounds: VExUS from @ThinkingCC / CCUS Institute on Vimeo.
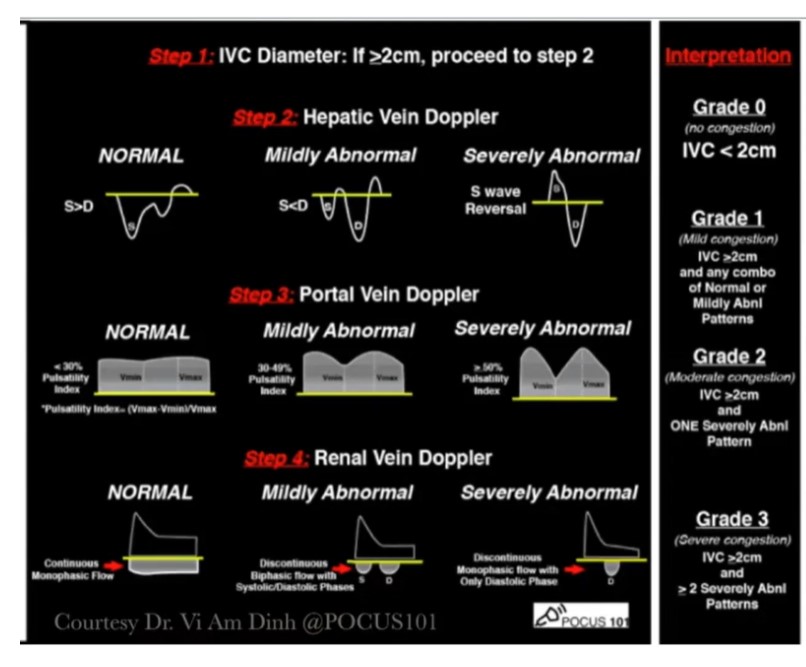
Here is the Vexus score chart:
Remember that Dr. Rola reminds you to evaluate the IVC diameter in both the long and the short-axis view.
The VExUS Score: