In this post I link to and excerpt from Rex DK, Boland CR, Dominitz JA, et al.
Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc.
2017;86(1):18-33. doi:10.1016/j.gie.2017.04.003 [PubMed Abstract] [Full-TextHTML] [Full-Text PDF].
All that follows is from the above resource.
Abbreviations:
CRC (colorectal cancer), FIT (fecal immunochemical test), MSTF (U.S. Multi-Society Task Force on Colorectal Cancer), SSP (sessile serrated polyp)
This document updates the colorectal cancer (CRC) screening recommendations of the U.S. Multi-Society Task Force of Colorectal Cancer (MSTF), which represents the American College of Gastroenterology, the American Gastroenterological Association, and The American Society for Gastrointestinal Endoscopy. CRC screening tests are ranked in 3 tiers based on performance features, costs, and practical considerations. The first-tier tests are colonoscopy every 10 years and annual fecal immunochemical test (FIT). Colonoscopy and FIT are recommended as the cornerstones of screening regardless of how screening is offered. Thus, in a sequential approach based on colonoscopy offered first, FIT should be offered to patients who decline colonoscopy. Colonoscopy and FIT are recommended as tests of choice when multiple options are presented as alternatives. A risk-stratified approach is also appropriate, with FIT screening in populations with an estimated low prevalence of advanced neoplasia and colonoscopy screening in high prevalence populations. The second-tier tests include CT colonography every 5 years, the FIT–fecal DNA test every 3 years, and flexible sigmoidoscopy every 5 to 10 years. These tests are appropriate screening tests, but each has disadvantages relative to the tier 1 tests. Because of limited evidence and current obstacles to use, capsule colonoscopy every 5 years is a third-tier test. We suggest that the Septin9 serum assay (Epigenomics, Seattle, Wash) not be used for screening. Screening should begin at age 50 years in average-risk persons, except in African Americans in whom limited evidence supports screening at 45 years. CRC incidence is rising in persons under age 50, and thorough diagnostic evaluation of young persons with suspected colorectal bleeding is recommended. Discontinuation of screening should be considered when persons up to date with screening, who have prior negative screening (particularly colonoscopy), reach age 75 or have <10 years of life expectancy. Persons without prior screening should be considered for screening up to age 85, depending on age and comorbidities.
Persons with a family history of CRC or a documented advanced adenoma in a first-degree relative age <60 years or 2 first-degree relatives with these findings at any age are recommended to undergo screening by colonoscopy every 5 years, beginning 10 years before the age at diagnosis of the youngest affected relative or age 40, whichever is earlier. Persons with a single first-degree relative diagnosed at ≥60 years with CRC or an advanced adenoma can be offered average-risk screening options beginning at age 40 years.
Recommendations
1. We recommend that clinicians offer CRC screening
beginning at age 50 (strong recommendation, highquality evidence). (See below for adjustments in recommended age for onset of screening based on race and family history.)
2. We suggest that sequential offers of screening tests, offering multiple screening options, and risk-stratified screening are all reasonable approaches to offering screening (weak recommendation, low-quality evidence).
Screening targets
The object of screening is to reduce CRC incidence and mortality. To accomplish both aims, tests need to detect early-stage (ie, curable) CRCs and high-risk precancerous lesions.1, 21
Detection and removal of precancerous lesions prevents CRC.30,31
The 2 main classes of precancerous lesions in the colon are conventional adenomas and serrated class lesions (Table 2). These 2 classes of precancerous lesions have distinct endoscopic features and histology and different (though overlapping) distributions within the colorectum. Specific screening tests sometimes have particular strengths or weaknesses detecting 1 or the other class of precancerous lesions, particularly the serrated class. Therefore, we review here the main clinical features of the 2 classes of precancerous lesions.
The adenoma–carcinoma sequence is believed to typically take more than 10 years to complete in sporadic cancers, whereas much shorter intervals occur in Lynch syndrome.34Correspondingly, colonoscopy is recommended at 10-year intervals in average-risk persons and at 1- to 2-year intervals in those with Lynch syndrome.1, 34The distribution of adenomas is relatively even throughout the colon, although adenomas with a flat or depressed morphology are distributed more to the proximal colon and pedunculated lesions more to the distal colon.35Adenomas are by definition dysplastic, with the overwhelming majority being low grade. The presence of high-grade dysplasia in an adenoma should be noted by a pathologist. Adenomas can also be characterized by tubular versus villous histology, with the overwhelming majority tubular. Lesions with >25% villous elements are termed tubulovillous and those with >75% villous elements villous.Villous elements and invasive cancer are associated with increasing size of adenomas.Invasive cancer in adenomas ≤5 mm in size is extremely rare, and the prevalence remains well below 1% in adenomas 6 to 9 mm in size.36 Recent colonoscopic studies have identified lower prevalence rates of cancer in polyps <1 cm in size compared with early studies, probably because improvements in colonoscope technology and performance have led to routine detection of an array of small, flat, low-volume adenomas.36Interobserver agreement in differentiation of high- versus low-grade dysplasia by pathologists and tubular versus tubulovillous histology is poor to moderate, particularly in adenomas <1 cm in size.37 Conversely, interobserver agreement between pathologists is good to excellent in placing lesions within the conventional adenomas versus serrated polyps and in identifying invasive cancer.38
An important clinical concept is the “advanced” adenoma, defined as a lesion ≥1 cm in size or having high-grade dysplasia or villous elements.3 Because nonadvanced adenomas have a very low prevalence of cancer and a long adenoma–cancer sequence, screening tests can remain useful if they target cancer and advanced adenomas and not small adenomas. Further, the prevalence of nonadvanced adenomas is so high in modern colonoscopy studies that detection of such lesions by noncolonoscopic screening tests leads to unacceptably low specificity. Colonoscopy has an important benefit over other screening methods because of its ability to detect and remove both advanced and nonadvanced adenomas. Although nonadvanced adenomas have limited clinical importance and are not the target of noncolonoscopic screening methods, colonoscopists strive to identify and remove nonadvanced adenomas. Thus, resecting lesions with any precancerous potential during colonoscopy is safe, seems to be better accepted by patients in the United States, and removes them as a clinical concern.
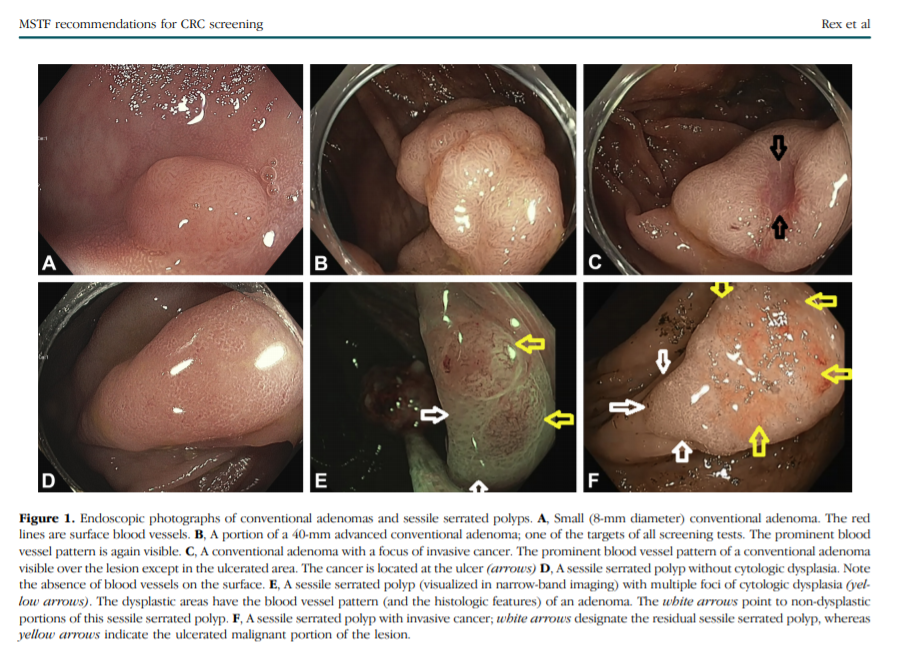
Serrated colorectal lesions (Fig. 1) represent an emerging area in the field of precancerous colorectal lesions.
The serrated class of precursor lesions accounts for up to 30% of CRCs.33
Within the serrated class, hyperplastic polyps are not currently considered precancerous, whereas sessile serrated polyps (SSPs; also known as sessile serrated adenoma) and traditional serrated adenomas are considered precancerous (Table 2).33
Hyperplastic polyps are usually small lesions and are distributed toward the distal colon.39
SSPs are common (found in 8%-9% of screening colonoscopies performed by expert detectors)40, 41 and are distributed toward the proximal colon compared with conventional adenomas. SSPs are typically flat or sessile in shape, have few or no surface blood vessels (conventional adenomas by comparison have many surface vessels), and are more difficult to detect at colonoscopy than conventional adenomas.33, 42, 43
Because of their prevalence and precancerous potential, SSPs are the major precancerous serrated lesion. There is poor interobserver agreement between pathologists in the differentiation of hyperplastic polyps fromSSPs.44 Consequently, clinicians can see widely varying rates of SSPs in pathology reports, depending on the pathologist or even the center in which they practice.45
Most SSPs are not dysplastic, and the lesions should consistently be designated as “SSP without cytologic dysplasia” or “SSP with cytologic dysplasia.”46
When a dysplastic component is present, it is often evident endoscopically (Fig. 1) and histologically is a region of conventional adenoma within an otherwise serrated lesion.47 Microdissection studies indicate that the dysplastic area often has microsatellite instability.48 The SSP with cytologic dysplasia is considered a more advanced lesion in the polyp cancer sequence than SSP without cytologic dysplasia.3, 33, 49, 50
The features of these 2 classes of precancerous lesions are relevant to the available screening tests. Colonoscopy is the criterion standard for the detection of all precancerous colorectal lesions. Colonoscopy achieves its greatest superiority relative to other screening tests in the detection of conventional adenomas <1 cm in size and serrated class lesions. Detection of SSPs is a major deficiency of flexible sigmoidoscopy because SSPs are predominantly in the proximal colon,51 of CT colonography because the lesions tend to be flat,52 and of FIT53 probably because SSPs have no or few surface blood vessels with less tendency to bleed than conventional adenomas.
The combined FIT–fecal DNA test achieves its greatest relative performance compared with FIT alone in the detection of serrated class lesions, related to the poor sensitivity of FIT for these lesions and the inclusion of hypermethylation markers in the DNA panel.53 Hypermethylation is a feature of serrated lesions.33
Specific screening tests
Colonoscopy
Disadvantages of colonoscopy include the need for thorough bowel cleansing, a higher risk of perforation relative to the other screening tests, higher risk of aspiration pneumonitis (particularly when the procedure is performed with deep sedation),69 a small risk of splenic injury requiring splenectomy, and a greater risk of postprocedural bleeding compared with other screening tests. A meta-analysis of population-based studies found risks of perforation, bleeding, and death of .5 per 1000, 2.6 per 1000, and 2.9 per 100,000, respectively.70 Bleeding after colonoscopy is almost entirely related to polypectomy. When electrocautery is used for resection of all colorectal polyps, most bleeds occur after resection of small lesions. This relates entirely to the high prevalence of these lesions because increasing polyp size and proximal colon location are the major risk factors for bleeding per individual resected polyp.71
Cold resection techniques are effective and nearly devoid of clinically significant bleeding risk and can be generally advised for nonpedunculated lesions <1 cm in size.72
Despite these risks, colonoscopy is the preferred approach to management of any benign colorectal polyp regardless of size or location because the alternative of surgical resection has higher mortality and cost compared with colonoscopy.73, 74
To the extent that other screening tests effectively identify large lesions, they result in colonoscopy and do not prevent adverse events related to colonoscopic resection of large lesions.
A major disadvantage of colonoscopy is operator dependence in performance. Operator dependence affects detection of cancer,67, 68, adenomas,76, 77 and serrated lesions40, 41, 78; selection of appropriate screening and surveillance intervals after colonoscopy79; and effective resection of colorectal polyps.80
In general, gastroenterologists performing colonoscopy are more effective than nongastroenterologists in prevention of cancer62, 81, 82, 83 and detection of precancerous polyps.84
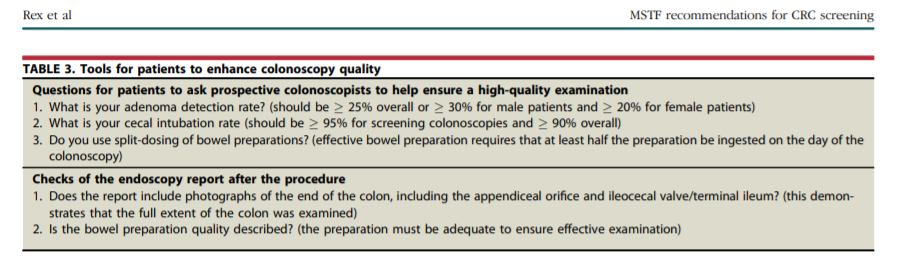
However, substantial operator dependence within gastroenterologists is consistently observed,42, 43, 76, 77, 78 so that selection of a colonoscopist by specialty is not adequate protection against suboptimal operator performance. Table 3 shows a list of questions that patients can ask potential colonoscopists to judge whether performance is likely to be at a high level. Afterward, the colonoscopy report should contain the items in Table 3 as an additional check on the adequacy of the procedure.
Fecal immunochemical test
Advantages of FIT include its noninvasive nature, 1-time sensitivity for cancer of 79% in 1 meta-analysis,85 fair sensitivity for advanced adenomas (approximately 30%), and low 1-time cost (approximately $20). FIT is recommended annually in the United States. The MSTF has recently issued detailed recommendations on the technical performance of FIT86 and considers FIT an essential element of the CRC screening armamentarium for all practitioners. FIT is commonly the test of choice in programmatic screening, an excellent second choice for practitioners using sequential testing who offer colonoscopy first, and should likely always be one of the tests included in a multiple-options approach. Disadvantages of FIT include the need for repeated testing, which can be problematic in the nonprogrammatic (opportunistic) setting,13 and poor or no sensitivity for serrated class precursor lesions.53 However, there is no evidence that cancers arising through serrated class lesions are less likely to bleed than those arising via adenomas.
FIT–fecal DNA test
The U.S. Food and Drug Administration (FDA) approved a CRC screening test that is a combination of a FIT and markers for abnormal DNA53 (Cologuard; Exact Sciences; Boston, Mass). The Center for Medicaid & Medicare Services approved the test for reimbursement and recommends performance at 3-year intervals. In a large screening colonoscopy study, patients underwent FIT, the combined FIT–fecal DNA test, and colonoscopy. The FIT–fecal DNA test had a 1-time sensitivity for CRC of 92%. The FIT assay tested in the study had 73.8% sensitivity for cancer, suggesting that most cancer sensitivity of the FIT–fecal DNA test can be achieved without addition of DNA markers. Advantages of the FIT–fecal DNA test include the highest single-time testing sensitivity for cancer of any noninvasive, nonimaging CRC screening test. Also, the study demonstrated 40% sensitivity for SSPs >1 cm in size. The sensitivity of FIT for SSPs was equal to the false-positive rate, indicating no sensitivity.
The major disadvantages of the FIT–fecal DNA test are a substantial decrease in specificity (86.6% in persons with normal colonoscopy or nonadvanced lesions and 89.8% in those with normal colonoscopy), compared with 96% for the FIT test alone, and high cost relative to FIT. Specificity decreased with increasing age and was only 83% in persons aged >65 years. The cost of the FIT–fecal DNA test is approximately $600 for privately insured patients and about $500 for Medicare patients, about 10 times the direct costs of annual FIT. Moreover, there is a further increase in relative costs related to higher numbers of colonoscopies per test. However, specificity of every 3-year testing with the FIT–fecal DNA test may be approximately equal to the anticipated specificity over 3 years of annual FIT testing. There is currently no information regarding the programmatic sensitivity of the FIT–fecal DNA test. Annual FIT is more effective and less costly than FIT–fecal DNA every 3 years,87 so the FIT–fecal DNA test is unlikely to replace FIT in large organized screening programs. The FIT–fecal DNA test could be particularly appropriate for patients in the 50- to 65-year age group who seek a noninvasive test with very high sensitivity for cancer, because the test has better specificity in this age group. Available evidence suggests that asymptomatic patients with a positive FIT–fecal DNA test and a negative high-quality colonoscopy do not need the colonoscopy repeated or evaluation of the remainder of the GI tract.
CT colonography
CT colonography has replaced double-contrast barium enema as the test of choice for colorectal imaging for nearly all indications. CT colonography is more effective than barium enema and better tolerated.88, 89 Advantages of CT colonography include a lower risk of perforation compared with colonoscopy and sensitivity of 82% to 92% for adenomas ≥1 cm in size.88, 89, 90, 91 Disadvantages of CT colonography include the use of bowel preparation in most centers in the United States. CT colonography can be performed with laxative-free protocols, but this results in clear reductions in sensitivity relative to colonoscopy,91 including for large polyps. The sensitivity of CT colonography for polyps <1 cm is less than colonoscopy,88, 89, 90,are major deficiencies of CT colonography. Detection of extracolonic findings by CT colonography is common, and these findings have been classified by the American College of Radiology according to their clinical relevance.94
Radiation exposure is generally viewed as a disadvantage of CT colonography.14 Evidence that CT colonography reduces CRC incidence or mortality is lacking.Even in centers where CT colonography has long been available, the impact of CT colonography is limited. At one university, after full development of a CT colonography program, CT colonography accounted for about 10% of colorectal imaging studies, even with the availability of insurance coverage.95, 96Primary care physicians view the need for frequent follow-up colonoscopy examinations and management of incidental extracolonic findings as major factors limiting the utility of CT colonography.96 In general, despite an extensive literature investigating the performance of CT colonography, the test has limited impact on CRC screening compliance.95 However, CT colonography appeals to a niche of patients who are willing to undergo bowel preparation and are concerned about the risks of colonoscopy. When used, the recommended interval is 5 years in patients with normal CT colonography. We continue to recommend that patients with polyps ≥6 mm in size at CT colonography undergo colonoscopy.1
Quality of screening
Variable performance of screening tests affects at least colonoscopy, sigmoidoscopy, CT colonography, and FIT. Optimal results in CRC screening cannot be achieved without optimizing the technical performance and reporting of tests and ensuring that patients undergo appropriate follow-up after testing. The MSTF has made detailed recommendations regarding the technical performance of FIT86 and has previously issued quality recommendations regarding the technical performance of sigmoidoscopy117 and colonoscopy.118 The recommendations of the MSTF regarding quality in the technical performance of colonoscopy118 were largely incorporated in quality recommendations from a combined American College of Gastroenterology–American Society for Gastrointestinal Endoscopy Task Force on quality in 2006119 and 2015,120 and the MSTF endorses the American College of Gastroenterology–American Society for Gastrointestinal Endoscopy Task Force recommendations.
The burden of performing high-quality FIT falls largely on primary care physicians and/or the healthcare systems in which they work. In the opportunistic setting there may not be resources allocated to systematically ensure that FIT-positive patients are referred for colonoscopy and that FIT-negative patients are offered repeat testing or to monitor whether compliance with quality targets is adequate.13 Inability to allocate resources to monitor the quality of FIT testing is a factor favoring reliance on sequential testing with colonoscopy the first test offered.
Unlike primary care physicians, the main role of gastroenterologists in the screening process is to perform colonoscopy on patients referred for primary colonoscopy screening or for colonoscopy to evaluate other positive screening tests. As such, a primary task of gastroenterologists is to perform high-quality colonoscopy and cost-effective follow-up. The adenoma detection rate, originally proposed by the MSTF in 2002,118 has emerged as the most important and highly variable measure of the quality of mucosal inspection during colonoscopy. Two large studies have validated the adenoma detection rate as a predictor of cancer prevention by colonoscopy.67, 68 Measurement of the adenoma detection rate is mandatory to appreciating whether a colonoscopist should be performing screening colonoscopy. Patients should expect a prospective colonoscopist to provide his or her adenoma detection rate, which should meet or exceed recommended minimum thresholds (Table 3).
Practical considerations
Start here.