In this post I link to and excerpt from Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Am Heart Assoc. 2020 Jul 7;9(13):e017559.
Here are direct links to the sections of the HTML article:
Jump to
Here are excerpts:
ABSTRACT: Direct oral anticoagulants (DOACs) have quickly become attractive alternatives to the long-standing standard of
care in anticoagulation, vitamin K antagonist. DOACs are indicated for prevention and treatment of several cardiovascular conditions. Since the first approval in 2010, DOACs have emerged as leading therapeutic alternatives that provide both clinicians and patients with more effective, safe, and convenient treatment options in thromboembolic settings. With the expanding role of DOACs, clinicians are faced with increasingly complex decisions relating to appropriate agent, duration of treatment, and use in special populations. This review will provide an overview of DOACs and act as a practical reference for clinicians to optimize DOAC use among common challenging scenarios. Topics addressed include (1) appropriate indications; (2) use in patients with specific comorbidities; (3) monitoring parameters; (4) transitioning between anticoagulant regimens; (5) major drug interactions; and (6) cost considerations.
__________________________________________________________
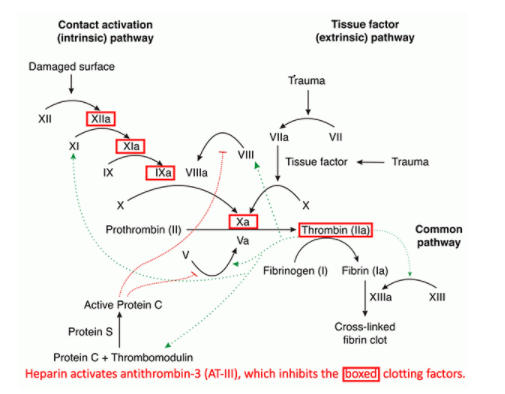
Figure below is from the outstanding Internet Book Of Critical Care’s chapter, Unfractionated heparin (UFH), LMWH, fondaparinux, argatroban, and bivalirudin, by Dr. Josh Farkas, May 24, 2020.
Direct oral anticoagulants (DOACs)—dabigatran
(Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis),
edoxaban (Savaysa), and betrixaban (Bevyxxa) are
anticoagulation pharmacotherapy used for the prevention of thrombosis in several cardiovascular contexts.1DOACs are categorized into 2 main classes:
- oral direct factor Xa inhibitors (ie, rivaroxaban, apixaban, edoxaban, and betrixaban) and
- direct thrombin inhibitors (ie, dabigatran)
DOACs are relatively new agents demonstrating superiority or noninferiority to prior standards of care, anticoagulation with vitamin K antagonists (VKA; ie, warfarin), or low-molecular-weight heparins (LMWHs), in reducing risk of thromboembolic complications with similar or reduced bleeding risk.2–5
The purpose of this review is to be a practical reference or algorithm for the busy clinician to navigate key aspects of effective DOAC prescribing, with an emphasis on addressing
key situations where clinical uncertainty exists. This review will provide recommendations to address special clinical situations to include indications, use in specific comorbidities, monitoring parameters, transitioning to or off of therapy, drug interactions, and cost.INDICATIONS FOR USE
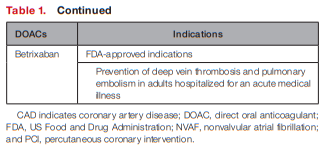
In general, FDA-approved indications for each of the
DOACs are comparable (see Table 1).
Dabigatran, rivaroxaban, apixaban, and edoxaban are approved for the lowering the risk of stroke and embolism in NVAF as well as deep vein thrombosis and pulmonary embolism
treatment/prophylaxis.8–11Unique indications include betrixaban for prophylaxis of venous thromboembolism (VTE) in hospitalized patients for an acute medical illness, and rivaroxaban in combination with aspirin to reduce major cardiovascular events in patients with chronic coronary artery disease (CAD) or peripheral artery disease.9,12
However, there is still uncertainty in understanding safe and effective use of DOACs in the setting of patients with cardiovascular comorbidities, specifically atrial fibrillation (AF) with recent percutaneous coronary intervention (PCI), AF with concomitant artificial heart valves, stable atherosclerotic cardiovascular disease (ASCVD), and cancer-associated thromboembolism. This section aims to clarify the use of DOACs within these patient subgroups.
AF and PCI
The combination of AF and CAD has often led to confusion regarding the optimal antithrombotic strategy, historically favoring atherothrombotic prevention to bleeding complications.
[See the rest of this section for details on the appropriate regimen.]
Another important consideration is the use of add-on therapy to further mitigate bleeding risk, specifically gastrointestinal bleeding risk in patients on dual therapy. As has been demonstrated in patients on DAPT, use of gastrointestinal protection with H2 antihistamine receptor blockers and proton pump inhibitors can reduce bleeding complications in patients
on DAPT.20Providers are encouraged to coprescribe gastrointestinal prophylaxis using a proton pump inhibitor for patients on dual-antithrombotic regimens, especially those at high risk of gastrointestinal bleeding. This same rationale may be applied to patients on DOACs and antiplatelets or agents with antiplatelet
properties (ie, NSAIDs, systemic corticosteroids, etc).AF and Artificial Heart Valves
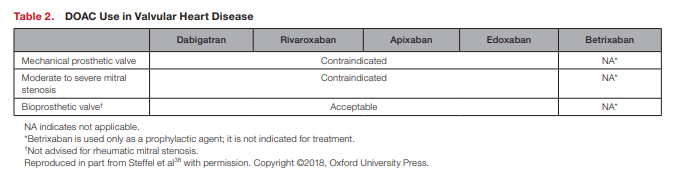
Valvular heart disease and AF commonly coexist and
each contribute independently to thromboembolic
events and mortality.21Valvular AF refers to the presence of mechanical prosthetic heart valve or moderate to severe mitral stenosis, conditions that substantially increase thromboembolic risk and serve as a principal exclusion criterion for the major DOAC phase III AF trials.22–25
More specifically, the RE-ALIGN (Dabigatran Versus Warfarin in Patients With Mechanical Heart Valves) trial established the use of DOACs as contraindicated in patients with mechanical heart valves.26 The study was terminated early, as patients with mechanical prosthetic heart valves experienced excess
thromboembolic and bleeding events. Therefore,
VKA remains the preferred agent for this patient
population as recommended in the current American
College of Chest Physicians and American College
of Cardiology/American Heart Association guidelines.27–29Bioprosthetic heart valves are considered less thrombogenic compared with mechanical heart valves but nonetheless are associated with increased risk and require chronic anticoagulation in the setting of concurrent AF or mitral stenosis.30
Overall, the use of DOACs in patients with AF with bioprosthetic
valves or other valve repairs still constitutes a gray area in clinical practice because of limited investigation and consensus about the definition of “valvular” AF.Despite the unfavorable outcomes of the RE-ALIGN
trial of mechanical valves, there is a growing interest
to investigate use of DOACs in the setting of bioprosthetic valves.[See this section for details supportive of the above. This section concludes with the following conclusion]:
Thus, the limited evidence supports the notion that bioprosthetic heart valves should not preclude DOAC use, a practice that is echoed by the European Heart Rhythm Association (see Table 2).38
Stable Atherosclerotic Cardiovascular
DiseaseAspirin use for secondary prevention of ASCVD is well
established and is widely recommended by major clinical guidelines for indefinite use.39 In this setting, major
ASCVD events are reduced ≈20% to 30%, but major
bleeding increased ≈1.4 to 1.6-fold.40,41In an effort to expand upon the ASCVD risk reduction witnessed
with aspirin monotherapy, prior investigations evaluated more potent antiplatelets (ie, clopidogrel, ticagrelor, and vorapaxar) and VKA therapy as alternatives to aspirin and as add-on therapy with aspirin.42 [But they were not helpful.][But] The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) study* demonstrated that rivaroxaban plus aspirin had significant secondary prevention benefits for patients with ASCVD (ie, CAD and peripheral artery disease), which led to FDA approval for this indication in 2018.44
*See text for details.
Overall, the COMPASS trial demonstrated significant benefit in reducing major cardiovascular events, a composite of cardiovascular death, stroke, and myocardial infarction by 24%
(P<0.001), and major adverse limb events (including amputations) by 46% (P=0.0037) with low doses of rivaroxaban in combination with aspirin.44,45 This novel indication for rivaroxaban provides an important new antithrombotic therapy regimen for a large group of high-risk patients with established ASCVD and another pathway for reducing residual atherosclerotic risk.A common clinical conundrum occurs in the setting of stable ASCVD and concomitant AF: What is the most effective long-term antithrombotic regimen?
This question was addressed in AFIRE
(Antithrombotic Therapy for Atrial Fibrillation With
Stable Coronary Disease).46[See text for details.]
The AFIRE trial was stopped early because of increased mortality in the combination therapy group (1.85% versus 3.37% per patient year; HR, 0.55; 95% CI, 0.38–0.81). The rivaroxaban monotherapy arm was noninferior in cardiovascular efficacy measures (P<0.001) and superior in
reducing major bleeding (P=0.01) compared with the
combination therapy group. Thus, the results of the
AFIRE study extended the concept of less intense antithrombotic for optimal risk-benefit established in the acute-care setting from PIONEER AF-PCI,16 RE-DUAL PCI,17 and AUGUSTUS,18 to the time period beyond a year from diagnosis and intervention.Cancer-Associated Thromboembolism*
[*See also Treatment of Malignancy Associated Venous Thromboembolism. May 06, 2020 | Randy K Ramcharitar, MD, MS ; Louise Man, MD; Aditya M sharma, MD. Expert Analysis from the American College of Cardiology.]
Patients with cancer are at increased risk for venous and arterial thromboembolism and bleeding events.48
Historically, LMWHs have been the cornerstone of cancer-associated VTE on the basis of 2 trials providing reduced thrombotic risk compared with warfarin.49,50
[The article references the ADAM VTE (Apixaban, Dalteparin, in Active Cancer Associated Venous Thromboembolism) and the Caravaggio (Apixaban for the Treatment of Venous Thromboembolism Associated With Cancer) trial support the use of apixiban.]
Thus, the current landscape for treatment of VTE in the cancer population seems to support apixaban over the current standard of care with LMWH and other DOACs, establishing the ideal balance of thrombosis prevention while not inducing excess bleeding.
In response [to the evidence], major guidelines now endorse
the use of DOAC in cancer-associated VTE treatment.55,56Trials investigating the use of DOACs for the prevention of VTE in patients with cancer have also been conducted and support the use of these agents in patients with cancer undergoing ambulatory chemotherapy with intermediate-high VTE risk
and low bleeding risk.48,57,588 As current practice heavily favors LMWH and VKAs, these novel and compelling trials supporting the use of DOACs will likely serve to ignite a paradigm shift in practice standards
among this growing population of complex patients.COMORBIDITIES AFFECTING DOAC PHARMACOKINETICS
Patient-specific comorbidities, particularly ones that affect systemic exposure of the medication, need to be taken into account when managing patients on DOAC therapy. Comorbidities may alter the DOAC elimination rate, therefore increasing the risk of a thromboembolic or bleeding events. Of the many factors that need to be considered, we will address
the 3 most pertinent patient comorbidities: renal impairment, hepatic impairment, and extreme body weights.Renal Insufficiency
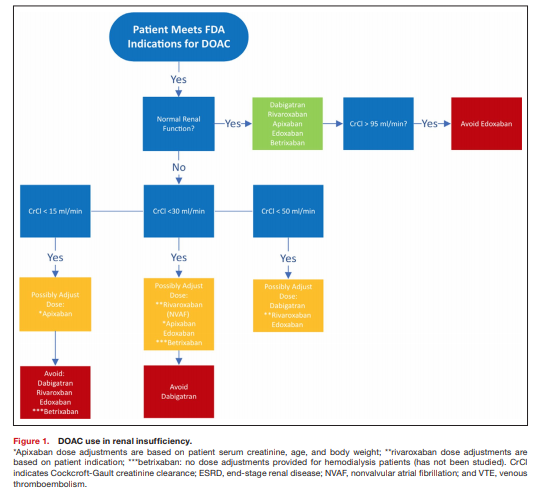
Patients with chronic kidney disease (CKD) have an increased risk for thromboembolic and bleeding events. Historically, warfarin has been the preferred anticoagulant used in severe CKD, but robust data supporting its efficacy and safety are lacking, coupled with the concern for warfarin-induced vascular calcifications and worsening nephropathy, more appropriate alternatives were eagerly sought.59
All DOAC therapies are eliminated by the kidneys to varying degrees, and alterations in renal clearance must be taken into account when dosing these agents.
Any clinical decisions on how to treat patients with DOACs requires the assessment of patient renal function, which should
be monitored frequently, at least annually or more frequently if a patient has additional comorbidities and risk factors. Renal dose adjustments include a decreased dose or decreased frequency of the regimen, and for most DOACs are based on the Cockcroft-Gault CrCl equation to estimate renal function.Phase III trials involving DOACs have excluded patients with severe renal dysfunction (CrCl <30 mL/min) or dialysis.22,23,25
In summary, DOACs are safe and effective in patients with moderate CKD (CrCl 30–50 mL/min). Dabigatran, rivaroxaban, and edoxaban should undergo dose adjustment for renal impairment and should be avoided for severe renal impairment (CrCl <30 mL/min). Edoxaban should be avoided in those with normal renal function (>95 mL/min). Apixaban and betrixaban undergo the least amount of renal elimination and may be the DOACs of choice in severe renal impairment. See Figure 1 for suggested DOAC use based on patient renal function.
Hepatic Impairment
Similar to other disease states noted above, patients with hepatic impairment are at increased risk of bleeding complications and thrombotic events.73 Alterations in hepatic function affects DOAC biotransformation to varying extents.
As there is no great monitoring parameter to assess for safety [of DOAcs], patients with hepatic dysfunction may not
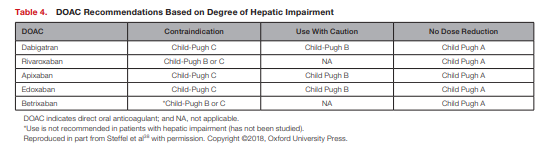
be ideal candidates for these agents. Restrictions for the use of DOACs in patients with hepatic impairment are based on the Child-Pugh classification system and exclusion criteria applied in pivotal trials (see Table 3).38,73,74
The Child-Pugh score uses the presence of clinical and biochemical abnormalities to assess the severity of the hepatic dysfunction.
All DOACs are contraindicated in patients with severe hepatic disease in which warfarin is the only recommended anticoagulant in this patient population.38
Dabigatran, apixaban, and edoxaban are viable options in patients with moderate hepatic impairment and do not require dose adjustments.38
All DOACs could be considered in patients with mild hepatic
impairment without any dose adjustments.With the lack of information, the optimal anticoagulation strategy for this patient population remains unclear and it is recommended to obtain blood tests to evaluate hepatic function and coagulation parameters before initiating and periodically throughout DOAC therapy.
Summarized in Table 4 are recommendations for patients with hepatic impairment based on their Child-Pugh classification.
Extreme Body Weights
Start here