In this post, I link to and excerpt from Excessive Daytime Sleepiness in Obstructive Sleep Apnea. Mechanisms and Clinical Management [PubMed Abstract] [Full-Text HTML] [Full-Text PMC Article] [Full-Text PDF]. Ann Am Thorac Soc. 2021 May;18(5):757-768.
There are 39 similar articles in PubMed Central.
The above article has been cited by five articles [See PubMed Abstract]
All that follows is from the above resource.
Abstract
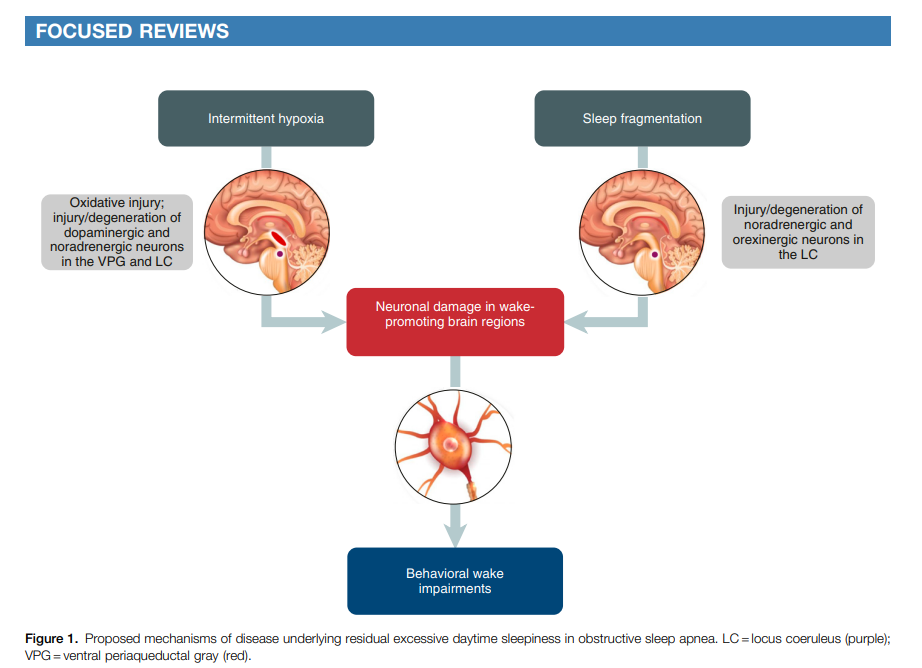
Many patients with obstructive sleep apnea (OSA) experience excessive daytime sleepiness (EDS), which can negatively affect daily functioning, cognition, mood, and other aspects of well-being. Although EDS can be reduced with primary OSA treatment, such as continuous positive airway pressure (CPAP) therapy, a significant proportion of patients continue to experience EDS despite receiving optimized therapy for OSA. This article reviews the pathophysiology and clinical evaluation and management of EDS in patients with OSA. The mechanisms underlying EDS in CPAP-treated patients remain unclear. Experimental risk factors include chronic intermittent hypoxia and sleep fragmentation, which lead to oxidative injury and changes in neurons and brain circuit connectedness involving noradrenergic and dopaminergic neurotransmission in wake-promoting regions of the brain. In addition, neuroimaging studies have shown alterations in the brain’s white matter and gray matter in patients with OSA and EDS. Clinical management of EDS begins with ruling out other potential causes of EDS and evaluating its severity. Tools to evaluate EDS include objective and self-reported assessments of sleepiness, as well as cognitive assessments. Patients who experience residual EDS despite primary OSA therapy may benefit from wake-promoting pharmacotherapy. Agents that inhibit reuptake of dopamine or of dopamine and norepinephrine (modafinil/armodafinil and solriamfetol, respectively) have demonstrated efficacy in reducing EDS and improving quality of life in patients with OSA. Additional research is needed on the effects of wake-promoting treatments on cognition in these patients and to identify individual or disorder-specific responses.
Clinical Evaluation of EDS in OSADifferential Diagnosis
When evaluating patients with OSA for residual EDS, healthcare providers should make individualized assessments of potential underlying causes of sleepiness (47).* The differential diagnosis includes areview for underrecognized comorbid conditions, such as sleep deprivation, idiopathic hypersomnia, narcolepsy, hypothyroidism, circadian rhythm disorders, psychiatric illness, chronic medical conditions, concomitant medications, or illicit drug use. Work schedules (in particular shift work), use of over-the-counter and prescribed sedating medications, and lifestyle factors also should be reviewed. Furthermore, healthcare
providers should ensure that the underlying airway obstruction is being adequately treated with CPAP, an oral appliance, hypoglossal nerve stimulation, or other surgical interventions (48–51).**
*47 . Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]**48 . Strollo PJ, Jr, Malhotra A. Stimulating therapy for obstructive sleep apnoea. Thorax. 2016;71:879–880. [PMC free article] [PubMed] [Google Scholar]Measures of EDS
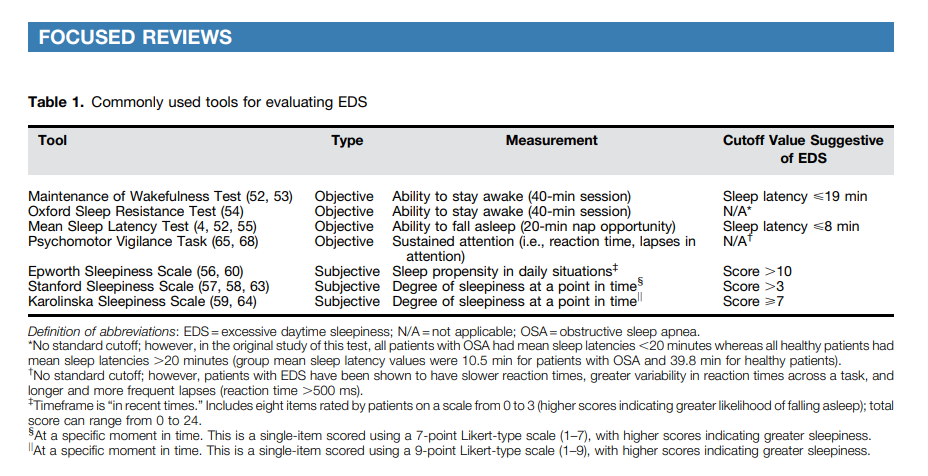
Clinical tools to evaluate EDS include objective and self-reported assessments of sleepiness, as well as assessments of cognition or alertness (Table 1).
Objective assessments for EDS include the Maintenance of Wakefulness Test (MWT), Oxford Sleep Resistance test, and MSLT (52). The MWT assesses a patient’s ability to stay awake for a defined time (a 40- min protocol is recommended) and
generally includes four trials performed at 2-hour intervals, beginning 1.5–3 hours after the patient’s usual wake-up time (52). A mean sleep latency <19 minutes on the 40-minute MWT has been suggested as a indicative of EDS (53). The Oxford Sleep Resistance test is structured similarly to the MWT but uses a
computerized method for monitoring wakefulness (individuals press a switch in response to a light-emitting diode) (54).
The MSLT assesses a patient’s ability to fall asleep, typically involving five nap opportunities (20 minutes each) at 2-hour
intervals, beginning 1.5–3 hours after termination of nocturnal PSG (52). A mean sleep latency <8 minutes is considered indicative of EDS (4, 55). These tests can be used during initial evaluation of patients with EDS, although the MSLT is more typically used when narcolepsy* (rather than OSA) is suspected (52). As they are time consuming and expensive, these tests may not be practical for screening or routine monitoring of treatment response unless there is some uncertainty as to the functional severity of EDS.
Self-reported measures of sleepiness include the Epworth Sleepiness Scale (ESS) (56), Stanford Sleepiness Scale (SSS) (57,
58), and Karolinska Sleepiness Scale (KSS) (59).[See text and footnotes for details.]
Treatment of EDS in OSA
Because EDS can persist in patients with OSA despite optimal treatment and can significantly impact QoL and increase risk of motor vehicle accidents, independent and direct management of EDS may be necessary in addition to primary OSA therapy. Pharmacologic management strategies for EDS should not replace primary treatment of the underlying airway obstruction.
Pharmacotherapy of EDS in OSA
Modafinil (PROVIGIL) (71), armodafinil (NUVIGIL) (72), and solriamfetol (SUNOSI) (73) are approved in the United
States for the treatment of EDS in adults with OSA; only solriamfetol is approved in the European Union for this indication (74). Modafinil and armodafinil bind to the dopamine transporter and inhibit dopamine reuptake (71, 72). Solriamfetol
binds to dopamine and norepinephrine transporters and inhibits reuptake of both neurotransmitters (73, 75). Thus, these three agents have pharmacologic actions on neurotransmitters implicated in sleep–wake regulation (76).
Several of these studies [of the above medications also
demonstrated improvements in QoL measures (Functional Outcomes of Sleep Questionnaire [FOSQ], Brief Fatigue
Inventory, and/or Short Form 36 [SF-36]) (78–80, 82).Common adverse events (AEs) associated with modafinil include headache, nausea, nervousness, rhinitis, diarrhea, back
pain, anxiety, insomnia, dizziness, and dyspepsia (71). Common AEs associated with armodafinil include headache, nausea,
dizziness, and insomnia (72). Rare but serious side effects (e.g., Stevens-Johnson Syndrome) can occur with modafinil/
armodafinil; drug interactions with CYP3A4/5 substrates, including oral contraceptives, are also a consideration
(71, 72).