Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Lancet. 2020 Jan 24. pii: S0140-6736(20)30183-5. Here are some excerpts:
In December, 2019, a series of pneumonia cases of unknown cause emerged in Wuhan, Hubei, China, with clinical presentations greatly resembling viral pneumonia.9
Thus far, more than 800 confirmed cases, including in health-care workers, have been identified in Wuhan, and several exported cases have been confirmed in other provinces in China, and in Thailand, Japan, South Korea, and the USA.10–13
Discussion
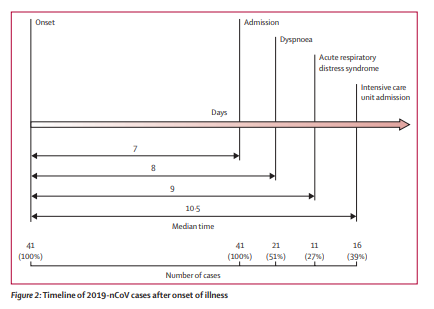
We report here a cohort of 41 patients with laboratoryconfirmed 2019-nCoV infection. Patients had serious, sometimes fatal, pneumonia and were admitted to the designated hospital in Wuhan, China, by Jan 2, 2020. Clinical presentations greatly resemble SARS-CoV. Patients with severe illness developed ARDS and required ICU admission and oxygen therapy. The time between hospital admission and ARDS was as short
as 2 days. At this stage, the mortality rate is high for 2019-nCoV, because six (15%) of 41 patients in this cohort died.The number of deaths is rising quickly. As of Jan 24, 2020, 835 laboratory-confirmed 2019-nCoV infections were reported in China, with 25 fatal cases. Reports have been released of exported cases in many provinces in China, and in other countries; some health-care workers have also been infected in
Wuhan. Taken together, evidence so far indicates human transmission for 2019-nCoV. We are concerned that 2019-nCoV could have acquired the ability for efficient human transmission.19 Airborne precautions, such as a fit-tested N95 respirator, and other personal protective equipment are strongly recommended.To prevent further spread of the disease in health-care settings that are caring for patients infected with 2019-nCoV, onset of fever and respiratory symptoms should be closely monitored among health-care workers. Testing of respiratory specimens should be done immediately once a diagnosis is suspected. Serum antibodies should be tested among health-care workers before and after their exposure to 2019-nCoV for identification of asymptomatic infections.
Similarities of clinical features between 2019-nCoV and
previous betacoronavirus infections have been noted. In
this cohort, most patients presented with fever, dry
cough, dyspnoea, and bilateral ground-glass opacities on
chest CT scans. These features of 2019-nCoV infection
bear some resemblance to SARS-CoV and MERS-CoV
infections.20,21 However, few patients with 2019-nCoV
infection had prominent upper respiratory tract signs
and symptoms (eg, rhinorrhoea, sneezing, or sore
throat), indicating that the target cells might be located in
the lower airway. Furthermore, 2019-nCoV patients rarely
developed intestinal signs and symptoms (eg, diarrhoea),
whereas about 20–25% of patients with MERS-CoV or
SARS-CoV infection had diarrhoea.21 Faecal and urine
samples should be tested to exclude a potential alternative
route of transmission that is unknown at this stage.