In this post I link to and excerpt from Therapeutic Management of Adults With COVID-19 from the National Institutes of Health, last updated Feb. 11, 2021.
Here are excerpts:
We are in the process of updating this section of the Guidelines to reflect the emerging data on the currently available anti-SARS-CoV-2 monoclonal antibodies. Until these updates are released, please see the COVID-19 Treatment Guidelines Panel’s Statement on the Emergency Use Authorization of Anti-SARS-CoV-2 Monoclonal Antibodies for the Treatment of COVID-19.
Executive Summary
Two main processes are thought to drive the pathogenesis of COVID-19. Early in the course of the infection, the disease is primarily driven by replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Later in the course of infection, the disease is driven by an exaggerated immune/inflammatory response to the virus that leads to tissue damage. Based on this understanding, it is anticipated that antiviral therapies would have the greatest effect early in the course of disease, while immunosuppressive/anti-inflammatory therapies are likely to be more beneficial in the later stages of COVID-19.
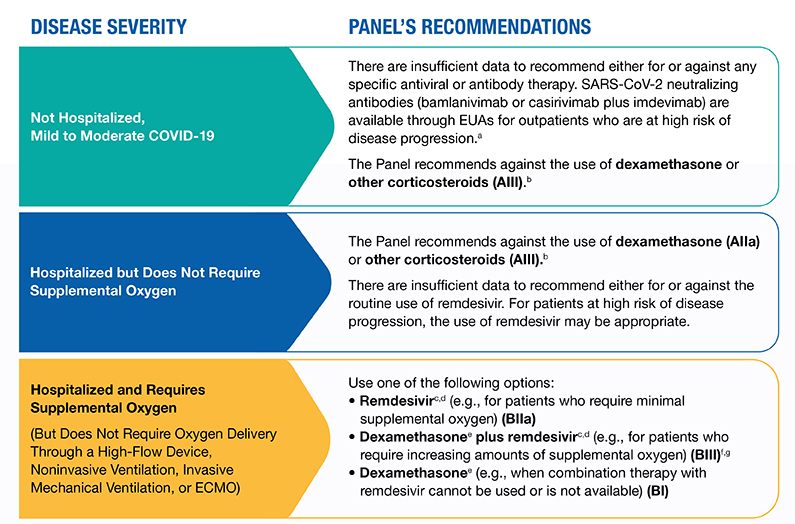
In the earliest stages of infection, before the host has mounted an effective immune response, anti-SARS-CoV-2 antibody-based therapies may have their greatest likelihood of having an effect. In this regard, although there are insufficient data from clinical trials to recommend either for or against the use of any specific therapy in this setting, preliminary data suggests that outpatients may benefit from receiving anti-SARS-CoV-2 monoclonal antibodies early in the course of infection. The Food and Drug Administration (FDA) has issued Emergency Use Authorizations (EUAs) for certain anti-SARS-CoV-2 monoclonal antibodies for the treatment of outpatients with mild to moderate COVID-19; please see Anti-SARS-CoV-2 Monoclonal Antibodies for more information.
Remdesivir, an antiviral agent, is currently the only drug that is approved by the FDA for the treatment of COVID-19. It is recommended for use in hospitalized patients who require supplemental oxygen. However, it is not routinely recommended for patients who require mechanical ventilation due to the lack of data showing benefit at this advanced stage of the disease.1-4
Dexamethasone, a corticosteroid, has been found to improve survival in hospitalized patients who require supplemental oxygen, with the greatest effect observed in patients who require mechanical ventilation. Therefore, the use of dexamethasone is strongly recommended in this setting.5-8
The COVID-19 Treatment Guidelines Panel (the Panel) continues to review the most recent clinical data to provide up-to-date treatment recommendations for clinicians who are caring for patients with COVID-19. Figure 1 summarizes the Panel’s recommendations for managing patients with varying severities of disease.