The following are excerpts from the article Management of Status Epilepticus in Children* [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Clin Med. 2016 Apr; 5(4): 47:
*The footnote numbers in the excerpt refer to the footnotes in the original article.
3. Epidemiology, Morbidity, and Mortality
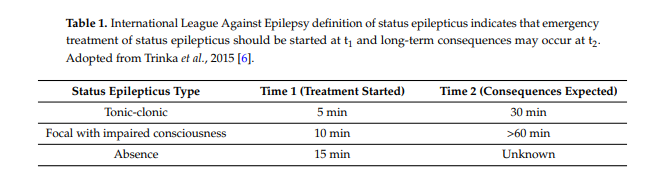
Current estimates of the incidence of status epilepticus in children vary by age. The incidence is highest in the neonatal period and declines until approximately five years of age [1,23–26]. Estimates in the neonatal to first year of life are approximately 135–150 incidents per 100,000 people [24,25],
with higher incidence in vulnerable populations with acute or chronic neurologic conditions. This
population also has a much higher incidence of acute symptomatic causes of status epilepticus [1,23]. The incidence of status epilepticus is relatively low between the ages of 5 and 40 years [24].4. Medical Management and Stabilization
Status epilepticus is a medical as well as neurologic emergency. Medical stabilization focuses on providing support of airway, breathing, and circulatory functions while identifying medical
complications and seizure precipitants. Medical management should proceed with subsequent testing once stabilization of airway, breathing, and circulation occurs. This may require intubation with mechanical ventilation to support pulmonary function and vasopressors and fluid resuscitation to support circulation. As hyperthermia [30] and hyperglycemia [31] are associated with unfavorable outcomes in some types of neurologic injury which may cause status epilepticus, close attention to these parameters is recommended. . . . . Steps to be completed in the “immediate” (initial five minute) time frame include non-invasive airway positioning, assessment of adequacy of ventilation and perfusion by checking vital signs, establishing a means of peripheral intravenous access, checking a finger-stick glucose, and checking a set of baseline triage labs. Once intravenous access is established, an “emergent” anti-seizure medication (i.e., a benzodiazepine) is administered. If intravenous access cannot be rapidly established, other routes of benzodiazepine administration should be used including intramuscular, rectal, buccal, and nasal.5. Diagnostics
Multiple studies have investigated pediatric status epilepticus etiologies, and febrile status epilepticus is the most common diagnosis [32–34]. Precipitant categories include acute symptomatic, remote symptomatic, acute-on-remote symptomatic, cryptogenic, and idiopathic. Acute symptomatic
and acute-on-remote symptomatic causes, which comprise 17% to 26% of cases of pediatric status epilepticus, respectively [23,32–34], should be evaluated urgently, as addressing these precipitants may simultaneously treat seizures. The American Academy of Neurology practice parameter addressing the diagnostic assessment of a child with convulsive status epilepticus reported that abnormal results among children who underwent testing included low anti-seizure medication levels (32%), neuroimaging abnormalities (8%), electrolytes (6%), inborn errors of metabolism (4%), ingestion (4%), central nervous system infections (3%), and positive blood cultures (3%) [35]. To identify these precipitants, the Neurocritical Care Society’s guideline recommends a finger-stick glucose in the
initial two minutes as well as a serum glucose, complete blood count, basic metabolic panel, blood gas, calcium, magnesium, and anti-seizure medication levels drawn in the initial five minutes [8]. Rapidly correctable causes of status epilepticus should be identified and treated as quickly as possible, including hypoglycemia, hypocalcemia, hyponatremia, and hypomagnesemia. Some patients may require additional diagnostic testing including lumbar puncture, neuroimaging, and other blood work (liver function tests, coagulation panel, serum or urine drug screen, inborn errors of metabolism screen), which are recommended to be performed in the initial hour. Testing for rarer causes of status epilepticus, including specific antibodies or PCR for viral encephalitides, autoantibody testing, or metabolic testing, may be considered in some patients [36].Consideration should also be given to performing continuous electroencephalogram (EEG) monitoring. The Neurocritical Care Society’s guideline stipulates that EEG monitoring should be initiated 15–60 min after seizure onset to evaluate for non-convulsive status epilepticus for patients who are not returning to baseline within 10 min of convulsive seizure cessation or within 60 min for patients in whom ongoing seizures are suspected.
6. Management of Status Epilepticus
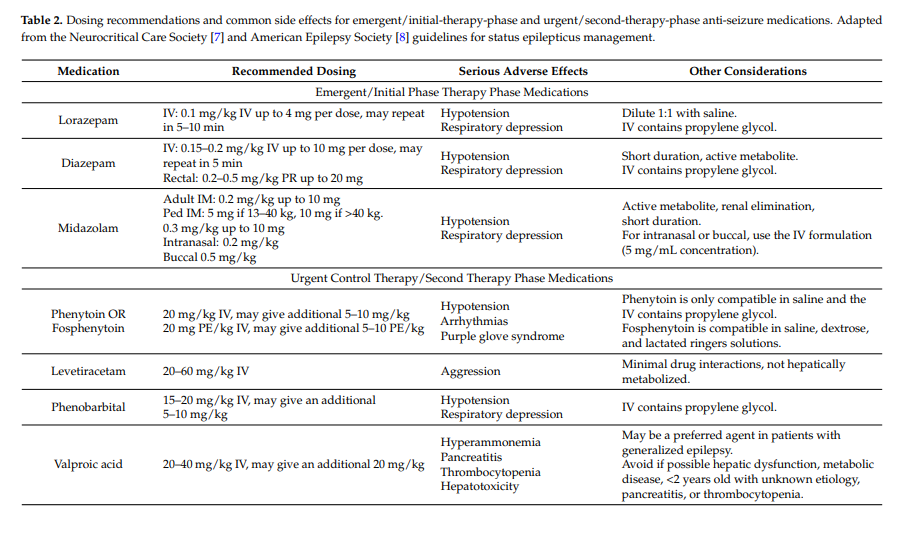
Administration of appropriate anti-seizure medications should occur as the patient is medically stabilized and diagnostic studies are performed. Table 2 provides a summary of recommended medications and doses.
The Neurocritical Care Society’s guideline states that benzodiazepines remain the “emergent initial therapy” of choice based both on available evidence and expert consensus. When possible, intravenous benzodiazepine administration is preferred. However, formulations exist for buccal, intranasal, intramuscular, and rectal administration, and these should be administered if intravenous access cannot be rapidly established.
Administration of benzodiazepines may result in respiratory depression and hypotension, so continued monitoring and stabilization should occur. The American Epilepsy Society’s guideline concludes that respiratory depression was the most common adverse event associated with anti-seizure medication treatment (level A evidence) and that there was no difference in respiratory depression between midazolam, lorazepam, and diazepam by any administration route (level B evidence) [9].
Second-line medications are referred to as “urgent” medications by the Neurocritical Care Society guideline [8] and “second therapy phase” by the American Epilepsy Society guideline [9]. If status epilepticus is already established, then benzodiazepines alone will obtain seizure control in less than half of children [19]. Thus, the Neurocritical Care Society’s guideline recommends that following benzodiazepine administration, another “urgent control medication” should be administered [8]. Only limited data are available regarding the comparative effectiveness of second-line “urgent” anti-seizure medications [65].
7. Management of Refractory Status Epilepticus
Refractory status epilepticus is characterized by seizures that persist despite treatment with adequate doses of initial anti-seizure medications. Definitions for refractory status epilepticus have varied in seizure durations (no time criteria, 30 min, one hour, or two hours) and/or lack of response to different numbers (two or three) of anti-seizure medications. The Neurocritical Care Society’s guideline indicates that refractory status epilepticus is diagnosed when clinical or electrographic
seizures persist after adequate doses of an initial benzodiazepine followed by a second appropriate anti-seizure medication [8].The Neurocritical Care Society’s guideline indicates that appropriate options for refractory status epilepticus management include administering a bolus of an unused “urgent” control medication and then proceeding to pharmacologic coma induction if seizures persist, or moving directly to pharmacologic coma induction [8]. . . . However, preparations should be initiated to achieve definitive seizure
control with continuous infusions. Substantial delays have been described before administration of pharmacologic coma induction in children with refractory status epilepticus indicating attention to timing is important [17].Few data are available regarding management of refractory status epilepticus with midazolam, pentobarbital, and other anesthetic therapies [96]. Midazolam dosing usually involves an initial loading dose of 0.2 mg/kg followed by an infusion at 0.05–2 mg/kg/hour titrated as needed to achieve clinical or electrographic seizure suppression or EEG burst-suppression. Pentobarbital dosing usually involves an initial loading dose of 5–15 mg/kg (followed by another 5–10 mg/kg if needed) followed by an infusion at 0.5–5 mg/kg/hour titrated as needed to achieve seizure suppression or EEG burst-suppression. If seizures persist with midazolam or pentobarbital, then escalating dosing through additional boluses is needed to rapidly increase levels and terminate seizures. Increasing the infusion rate without additional bolus dosing will lead to very slow increase in serum levels, which is inconsistent with the goal of rapid seizure termination. Anesthetics such as isoflurane are
also effective in inducing a burst-suppression pattern and terminating seizures but often lead to hypotension, and there are fewer data describing their use. Propofol may rapidly terminate seizures and induce burst-suppression, but it is rarely used in children due to its Federal Drug Administration black box warning due to propofol infusion syndrome.Patients treated with continuous infusions or inhaled anesthetics require intensive monitoring due to problems including: (1) mechanical ventilation for airway protection and to maintain appropriate oxygenation and ventilation; (2) central venous access and arterial access due to frequent laboratory tests and the possibility of developing hypotension requiring vasopressor or inotropic support; (3) temperature management since high dose sedatives and anesthetics can blunt the shivering response and endogenous thermoregulation; (4) assessment for development of lactic acidosis, anemia, thrombocytopenia, and end organ dysfunction (e.g., acute hepatic or renal injury); and (5) the risk of secondary infections due to indwelling catheters (e.g., central catheters, endotracheal tubes, and foley catheters), as well as some medications (e.g., pentobarbital).
It remains unclear whether the EEG treatment goal should be termination of seizures or induction of burst-suppression. The Neurocritical Care Society’s guideline considers either electrographic seizure cessation of burst-suppression as appropriate goals [8].
Resources:
(1) Management of Status Epilepticus in Children [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Clin Med. 2016 Apr; 5(4): 47:
(2) Guidelines for the evaluation and management of status epilepticus [PubMed Abstract] [Full Text PDF]. Neurocrit Care. 2012 Aug;17(1):3-23. doi: 10.1007/s12028-012-9695-z.
(3) Consensus statement on continuous EEG in critically ill adults and children, part I: indications [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Clin Neurophysiol. 2015 Apr;32(2):87-95. doi: 10.1097/WNP.0000000000000166.
(4) Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Clin Neurophysiol. 2015 Apr;32(2):96-108. doi: 10.1097/WNP.0000000000000165.