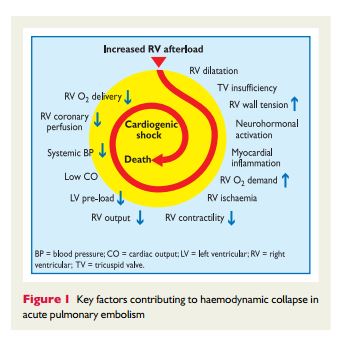
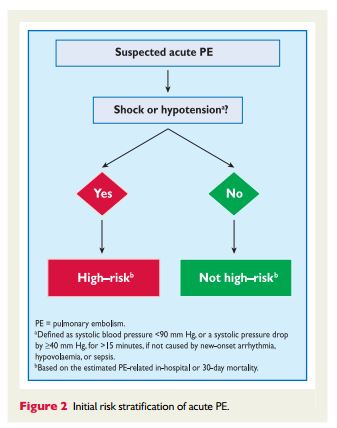
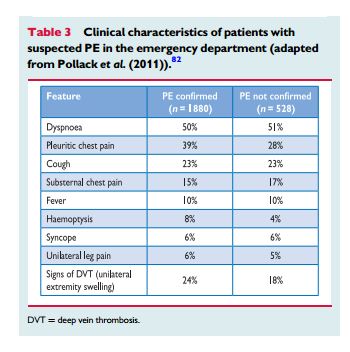
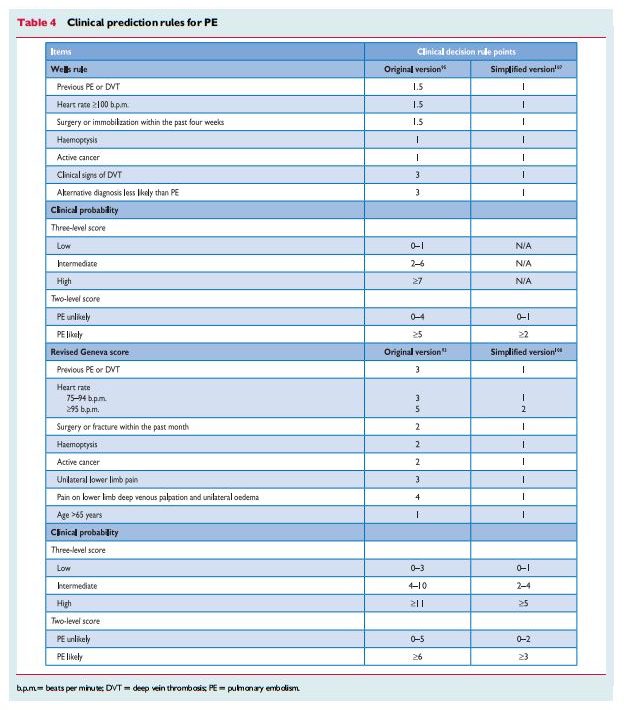
The appropriate diagnosis of pulmonary embolus depends on clinical acumen (informed pretest probability) and appropriate laboratory tests and imaging test. In addition to Reference (2), ACUTE PULMONARY EMBOLISM (DIAGNOSIS AND MANAGEMENT OF) ESC Clinical Practice Guidelines, there are two additional resources on Venous Thrombosis and Pulmonary Embolus in Additional Resources.
All that follows are excerpts from Reference (2):
3. Diagnosis
Throughout these Guidelines and for the purpose of clinical management, ‘confirmed PE’ is defined as a probability of PE high enough to indicate the need for PE-specific treatment, and ‘excluded PE’ as a probability of PE low enough to justify withholding PE-specific treatment with an acceptably low risk.
3.1 Clinical presentation
PE may escapeprompt diagnosis since the clinical signs and symptoms are non-specific (Table 3). When the clinical presentation raises the suspicion of PE in an individual patient, it should prompt further objective testing. In most patients, PE is suspected on the basis of dyspnoea, chest pain, pre-syncope or syncope, and/or haemoptysis.81–83 Arterial hypotension and shock are rare but important clinical presentations, since they indicate central PE and/or a severely reduced haemodynamic reserve. Syncopeisinfrequent,but may occur regardless of the presence of haemodynamic instability.84 Finally, PE may be completely asymptomatic and be discovered incidentally during diagnostic work-up for another disease or at autopsy.
3.2 Assessment of clinical probability
Despite the limited sensitivity and specificity of individual symptoms, signs, and common tests, the combination of findings evaluated by clinical judgement or by the use of prediction rules allows to classify patients with suspected PE into distinct categories of clinical or pre-test probability that correspond to an increasing actual prevalence of confirmed PE. As the post-test (e.g. after computed tomography) probability of PE
depends not only on the characteristics of the
diagnostic test itself but also on pretest probability, this has become a key step in all diagnostic algorithms for PE.3.4 Computed tomographic pulmonary angiography
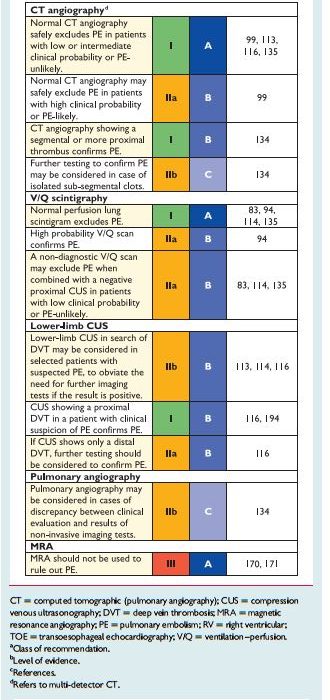
Taken together, these data [data reviewed in Section 3.4] suggest that a negative MDCT is an adequate criterion for excluding PE in patients with a non-high clinical probability of PE. Whether patients with a negative CT and a high clinical probability should be further investigated is controversial. MDCT showin PE at the segmental or more proximal level is adequate proof of PE in patients with a non-low clinical probability; however, the positive predictive value of MDCT is lower in patients with a low clinical probability of PE, and further testing may be considered, especially if the clots are limited to segmental or sub-segmental arteries.
The sensitivity and specificity of MDCT pulmonary angiography is a moving target. I recommend consulting your pulmonologist or interpreting radiologist as to what the sensitivity – specificity of the CT scanner that your patient will be evaluated with.
3.10 Diagnostic strategies
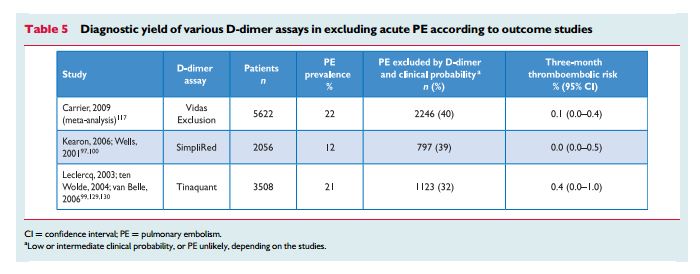
The prevalence of confirmed PE in patients undergoing diagnostic work-up because of suspicion of disease has been rather low (10–35%) in large series.99,100,113,116,197 Hence, the use of diagnostic algorithms is warranted, and various combinations of clinical assessment,plasma D-dimer measurement, and imaging tests have been proposed and validated. Thesestrategies were tested in patients presenting
with suspected PE in the emergency ward,99,113,114,116,197 during the hospital stay and more recently in the primary care setting.118,126 Failure to comply with evidence-based diagnostic strategies when withholding anticoagulation was associated with a significant increase in the number of VTE episodes and sudden cardiac death at threemonth
follow-up.198 The most straightforward diagnostic algorithms
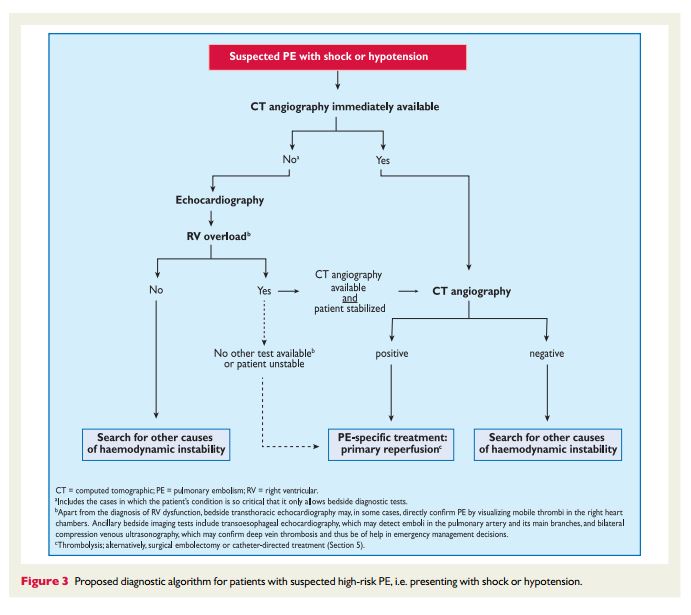
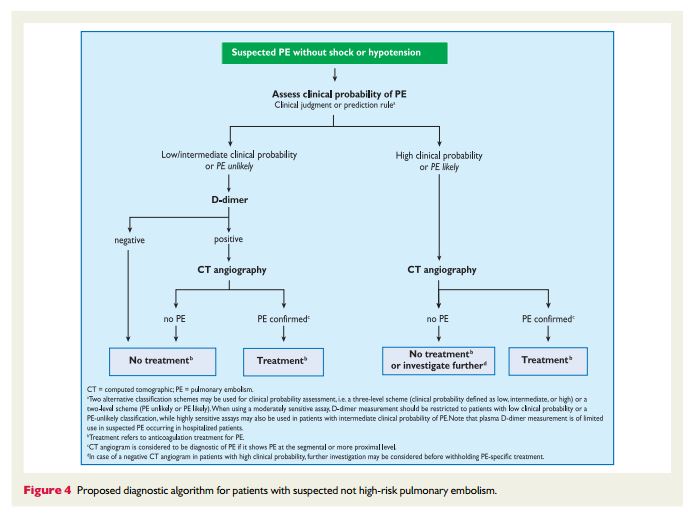
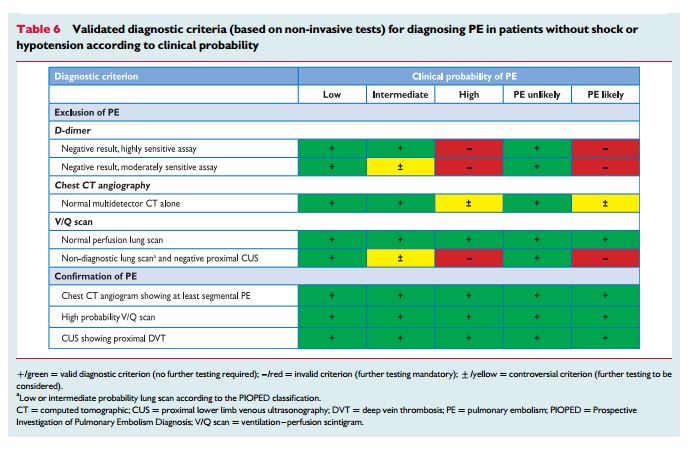
forsuspectedPE—with andwithoutshockor hypotension—are presented in Figures 3 and 4, respectively; however, it is recognized that the diagnostic approach to suspected PE may vary, depending on the availability of—and expertise in—specific tests in various hospitals and clinical settings. Accordingly, Table 6 provides the necessary
evidence for alternative evidence-based diagnostic algorithms.The diagnostic strategy for suspected acute PE in pregnancy is discussed in Section 8.1.
3.10.1 Suspected pulmonary embolism with shock
or hypotensionThe proposed strategy is shown in Figure 3. Suspected high-risk PE is an immediately life-threatening situation, and patients presenting with shock or hypotension present a distinct clinical problem. The clinical probability is usually high, and the differential diagnosis includes acute valvular dysfunction, tamponade, acute coronary syndrome (ACS), and aortic dissection. The most useful initial test in this situation is
bedside transthoracic echocardiography, which will yield evidence of acute pulmonary hypertension and RV dysfunction if acute PE is the cause of the patient’s haemodynamic decompensation. In a highly unstable patient, echocardiographic evidence of RV dysfunction is sufficient to prompt immediate reperfusion without further testing. This
decision may be strengthened by the (rare) visualization of right heart thrombi.184,199,2003.10.2 Suspected pulmonary embolism without shock
or hypotensionStrategy based on computed tomographic angiography (Figure 4) Computed tomographic angiography has become the main thoracic imaging test for investigating suspected PE but, since most patients with suspected PE do not have the disease, CT should not be the first-line test.
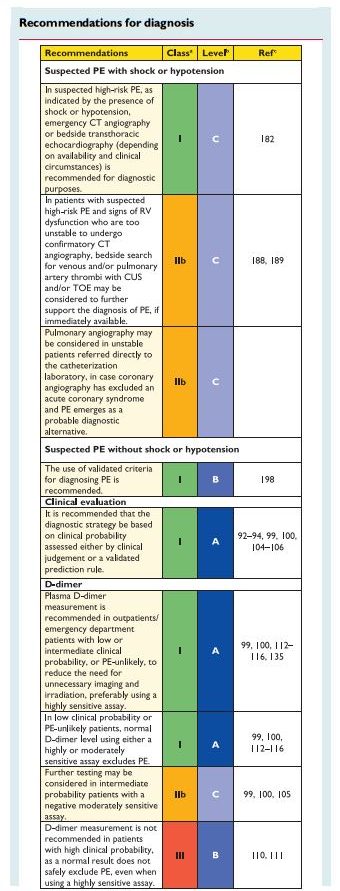
In patients admitted to the emergency department, plasma
D-dimer measurement, combined with clinical probability assessment, is the logical first step and allows PE to be ruled out inaround 30% of patients, with a three-month thromboembolic riskin patients left untreated of ,1%. D-dimer should not be measuredin patients with a high clinical probability, owing to a low negative predictivevalue in this population.202 It is also less useful in hospitalized patients because the number needed to test to obtain a clinically relevant negative result is high.In most centres, MDCT angiography is the second-line test in
patients with an elevated D-dimer level and the first-line test in
patients with a high clinical probability. CT angiography is considered to be diagnostic of PE when it shows a clot at least at the segmental level of the pulmonary arterial tree. False-negative results of MDCT have been reported in patients with a high clinical probabilityof PE;134 however, this situation is infrequent, and the three-month thromboembolic risk was low in these cases.99 Therefore, both the necessity of performing further tests and the nature of these tests in such patients remain controversial.
In view of the above, if I had a patient with a negative CT angiogram in the face of high clinical probability of PE, I would consult my pulmonologist to see what her/his opinion is as to the advisability of further workup and the advisability of anticoagulation.
Value of lower limb compression ultrasonography
Under certain circumstances, CUS can still be useful in the
diagnostic work-up of suspected PE. CUS shows a DVT in 30–50% of patients with PE,116,192,193 and finding proximal DVT in a patient suspected of PE is sufficient to warrant anticoagulant treatment without further testing.194 Hence, performing CUS before CT may be an option in patients with relative contraindications for CT such as in renal failure, allergy to contrast dye, or pregnancy.195,1964. Prognostic assessment
4.1 Clinical parameters
Various prediction rules based on clinical parameters have been
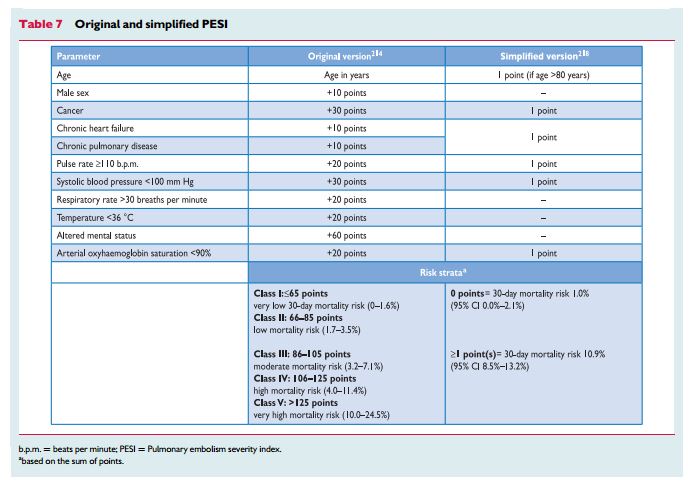
shown to be helpful in the prognostic assessment of patients with acute PE. Of those, the pulmonary embolism severity index (PESI; Table 7) is the most extensively validated score to date.211 – 214 Inone study,215 the PESI performed better than the older Geneva prognostic score216 for identification of patients with an adverse 30-day outcome. The principal strength of the PESI lies in the reliable identificationof patients at low risk for 30-day mortality (PESI Class I and II).
One randomized trial employed a low PESI as the inclusion criterion for home treatment of acute PE.217Owing to the complexityof the original PESI, which includes 11 differently weighted variables, a simplified version known as sPESI (Table 7) has been developed and validated.218,219 In patients with PE, the sPESI was reported to quantify their 30-day prognosis better than the shock index (defined as heart rate divided by systolic BP),220 and a simplified PESI of 0 was at least as accurate for identification of low-risk patients as the imaging parameters and laboratory biomarkers proposed by the previous ESC Guidelines.221 Combination of the sPESI with troponin testing provided additional prognostic information,222 especially for identification of low-risk patients.76
4.2 Imaging of the right ventricle by echocardiography or computed tomographic angiography
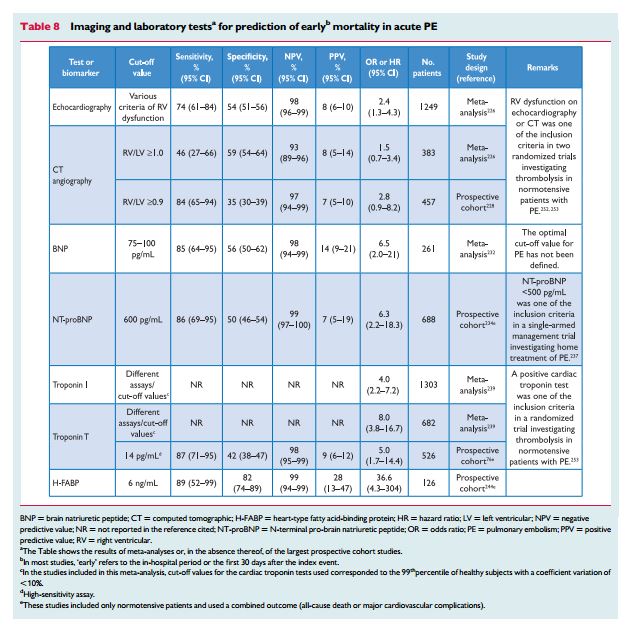
Echocardiographic findings indicating RV dysfunction have been reported in ≥25% of patients with PE.223 They have been identified as independent predictors of an adverse outcome,224 but are heterogeneous and have proven difficult to standardize.225 Still, in haemodynamically stable, normotensive patients with PE, echocardiographic assessment of themorphology and function of the RV may help in prognostic stratification.
As already mentioned in the previous section on the diagnosis of PE, echocardiographic findings used to risk stratify patients with PE include RV dilation, an increased RV–LV diameter ratio, hypokinesia of the free RV wall, increased velocity of the jet of tricuspid regurgitation, decreased tricuspid annulus plane systolic excursion, or combinations of the above. Meta-analyses have shown that RV dysfunction detected by echocardiography is associated with an elevated risk of short-term mortality in patients without haemodynamic instability, but its overall positive predictive value is low (Table 8).226,227 In addition to RV dysfunction, echocardiography can also identify right-to-left shunt through a patent foramen ovale and the presence of right heart thrombi, both of which are associated with increased mortality in patients with acute PE.80,184
CT angiography has also been used to demonstrate RV enlargement.
It is important to remember that all of the above are only excerpts from an incredibly complete and understandable guideline. It is worth reviewing the complete guideline.
Additional Resources:
(1) 2016 Antithrombotic Therapy for VTE Disease From Chest [The Links that follow are to the article in Chest: PubMed Abstract, Full Text HTML, Full Text PDF]
Posted on March 26, 2016 by Tom Wade MD
(2) ACUTE PULMONARY EMBOLISM (DIAGNOSIS AND MANAGEMENT OF)
ESC Clinical Practice Guidelines [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Eur Heart J. 2014 Nov 14;35(43):3033-69, 3069a-3069k. doi: 10.1093/eurheartj/ehu283. Epub 2014 Aug 29.
Erratum in
- Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. [Eur Heart J. 2015]
- Corrigendum to: 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. [Eur Heart J. 2015]
(3) 2013 NICE List of Quality Statements (Guidelines) for Diagnosis + Management of Venous Thromboembolic Disease Posted on July 30, 2014 by Tom Wade MD