In this post I link to the CDC’s Interim Guidelines for COVID-19 Antibody Testing. which instructs clinicians in the correct use of COVID-19 antibody test based on current scientific knowledge. This CDC page was last updated May 23, 2020 at the time of this post. CDC states that:
Data that will inform serologic testing guidance is rapidly evolving. Recommendations on the use of serologic tests to determine protective immunity and infectiousness among persons recently infected with SAR-CoV-2 will be updated as new information becomes available.
Here are direct links to all of the full sections of the Guidelines:
- Background
- Development of Antibodies and Immunity
- Current Status of Antibody Testing in the United States
- Types of Antibody Testing
- Optimizing Testing Outcomes
- Limitations
- Recommendations for Use
I excerpt from the Guidelines because it helps me reinforce my learning of the subject matter.
The section, Optimizing Testing Outcomes, of the Interim Guidelines is, I think, the most important section in entire Guidelines.
There are two types of COVID-19 Tests:
- The PCR viral test is to see if you currently have the COVID-19 infection
- I discuss this test in my post Do You Have An Acute COVID-19 Infection – The COVID-19 PCR Viral Test
- The bottom line from the above resource: “False negative PCR testing for SARS-CoV-2 is common! Each patient must be evaluated based on symptoms, timing, and other relevant/available clinical information. Don’t assume that a negative PCR means the patient is free from infection.”
- In this post I link to CDC’s Interim Guidelines for COVID-19 Antibody Testing. The CDC will be continuously revising the guidelines as new information becomes available.
- The purpose of this test is to determine if you have had a COVID-19 infection in the past.
- There are many factors to consider in using any of the many different antibody tests. All of these factors are well described in the above link which the CDC will be updating as new information becomes available.
- I discuss this test in my post Do You Have An Acute COVID-19 Infection – The COVID-19 PCR Viral Test
Here are excerpts from the CDC’s Interim Guidelines for COVID-19 Antibody Testing:
Summary
Serologic methods have been developed and will have important public health and clinical uses to monitor and respond to the COVID-19 pandemic.
- Serologic assays for SARS-CoV-2 now have Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA), which has independently reviewed their performance.
- Currently, there is no identified advantage of assays whether they test for IgG, IgM and IgG, or total antibody.
- It is important to minimize false positive test results by choosing an assay with high specificity and by testing populations and individuals with an elevated likelihood of previous exposure to SARS-CoV-2. Alternatively, an orthogonal testing algorithm (i.e., employing two independent tests in sequence when the first test yields a positive result) can be used when the expected positive predictive value of a single test is low.
- Antibodies most commonly become detectable 1-3 weeks after symptom onset, at which time evidence suggests that infectiousness likely is greatly decreased and that some degree of immunity from future infection has developed. However, additional data are needed before modifying public health recommendations based on serologic test results, including decisions on discontinuing physical distancing and using personal protective equipment.
Background
Unlike viral direct detection methods such as nucleic acid amplification or antigen detection tests that can detect acutely infected persons, antibody tests help determine whether the individual being tested was ever infected—even if that person never showed symptoms.
Serologic tests detect waning or past SARS-CoV-2 virus infection indirectly, by measuring the host humoral immune response to the virus. Therefore, serology assays do not typically replace direct detection methods as the primary tool for diagnosing an active SARS-CoV-2 infection, but they do have several important applications in monitoring and responding to the COVID-19 pandemic.
Although serologic tests should not be used at this time to determine if an individual is immune, these tests can help determine the proportion of a population previously infected with SARS-CoV-2 and provide information about populations that may be immune and potentially protected.
Development of Antibodies and Immunity
Nearly all immune competent individuals will develop an immune response following SARS-CoV-2 infection. Like infections with other pathogens, SARS-CoV-2 infection elicits development of IgM and IgG antibodies, which are the most useful for assessing antibody response because little is known about IgA response in the blood.
Antibodies in some persons can be detected within the first week of illness onset. SARS-CoV-2 infections are somewhat unusual because IgM and IgG antibodies arise nearly simultaneously in serum within 2 to 3 weeks after illness onset. Thus, detection of IgM without IgG is uncommon. How long IgM and IgG antibodies remain detectable following infection is not known.
Recurrence of COVID-19 illness appears to be very uncommon, suggesting that the presence of antibodies could confer at least short-term immunity to infection with SARS-CoV-2.
However, definitive data are lacking, and it remains uncertain whether individuals with antibodies (neutralizing or total) are protected against reinfection with SARS-CoV-2, and if so, what concentration of antibodies is needed to confer protection.
Current Status of Antibody Testing in the United States
FDA-authorized serologic tests
FDA now requires commercially marketed serologic tests to receive Emergency Use Authorization (EUA).
A list of all tests authorized for emergency use under EUA is maintained on an FDA website. All currently authorized tests are qualitative (providing a result that is positive, negative, or indeterminate) rather than quantitative (providing a quantitative assessment of antibody levels).
Both laboratory and rapid serologic assays have received EUA. Serologic testing technologies include single-use, low throughput lateral flow tests where the presence of antibody is demonstrated by a color change on a paper strip and laboratory-based immunoassays that allow for processing of many samples at the same time.
The EUA letter of authorization includes the settings in which the test is authorized, based on FDA’s determination of appropriate settings for use during the public health emergency.
Optimizing Testing Outcomes
Test performance
The utility of tests depends on the sensitivity and specificity of the assays; these performance characteristics are determined by using a defined set of negative and positive samples.
In addition, the predictive values of a test should be considered because these values affect the overall outcome of testing.
Positive predictive value is the probability that individuals with positive test results are truly antibody positive. Negative predictive value is the probability that individuals with negative test results are truly antibody negative. Positive and negative predictive values are determined by the percentage of truly antibody positive individuals in the tested population (prevalence, pre-test probability) and the sensitivity and specificity of the test. For example:
- In a high-prevalence setting, the positive predictive value increases — meaning it is more likely that persons who test positive are truly antibody positive – than if the test is performed in a population with low-prevalence. When a test is used in a population where prevalence is low, the positive predictive value drops because there are more false-positive results, since the pre-test probability is low.
- Likewise, negative predictive value is also affected by prevalence. In a high-prevalence setting, the negative predictive value declines whereas in a low-prevalence setting, it increases.
In most of the country, including areas that have been heavily impacted, the prevalence of SARS-CoV-2 antibody is expected to be low, ranging from <5% to 25%, so that testing at this point might result in relatively more false positive results and fewer false-negative results.
In some settings, such as COVID-19 outbreaks in food processing plants and congregate living facilities, the prevalence of infection in the population may be significantly higher. In such settings, serologic testing at appropriate intervals following outbreaks might result in relatively fewer false positive results and more false-negative results.
Testing strategies
In the current pandemic, maximizing specificity and thus positive predictive value in a serologic algorithm is preferred in most instances, since the overall prevalence of antibodies in most populations is likely low. For example, in a population where the prevalence is 5%, a test with 90% sensitivity and 95% specificity will yield a positive predictive value of 49%. In other words, less than half of those testing positive will truly have antibodies. Alternatively, the same test in a population with an antibody prevalence exceeding 52% will yield a positive predictive greater than 95%, meaning that less than one in 20 people testing positive will have a false positive test result.
Three strategies can be used to improve positive predictive value:
- Choosing a test with a very high specificity, perhaps 99.5% or greater, will yield a high positive predictive value in populations tested with prevalence >5%.
- Another strategy is to focus testing on persons with a high pre-test probability of having SARS-CoV-2 antibodies, such as persons with a history of COVID-19-like illness.
- A third approach is to employ an orthogonal testing algorithm in which persons who initially test positive are tested with a second test. Effective orthogonal algorithms are generally based on testing a patient sample with two tests, each with unique design characteristics (e.g., antigens or formats).
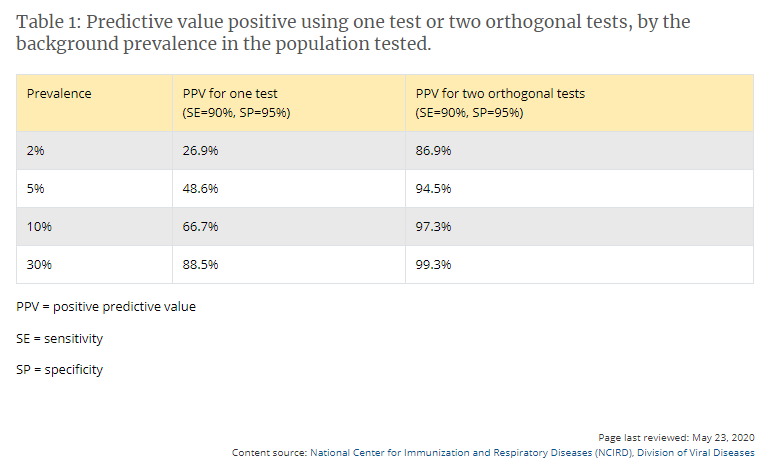
Algorithms can be designed to maximize overall specificity while retaining maximum sensitivity. For example, in the example above with a population prevalence of 5%, a positive predictive value of 95% can be achieved if samples initially positive are tested with a second different orthogonal assay that also has 90% sensitivity and 95% specificity. The performance of orthogonal testing algorithms has not been systematically evaluated but can be estimated using an on-line calculatorexternal icon from the FDA. See Table 1 for the potential improvement benefits of the orthogonal testing algorithm.
Limitations of Serologic Tests
At present, the immunologic correlates of immunity from SARS-CoV-2 infection are not well defined. Representatives from BARDA, CDC, FDA, NIH, the Office of the Assistant Secretary for Health (OASH), Department of Defense (DoD), and White House Office of Science and Technology Policy (OSTP) are working with members of academia and the medical community to determine whether positive serologic tests are indicative of protective immunity against SARS-CoV-2.
The kinetics of antibody response, longevity of antibodies, the ability of antibodies to protect from repeat infection, the protective titer of neutralizing antibody, and the correlation of binding antibody titers to neutralization ability are yet to be determined.
Hence, pending additional data, the presence of antibodies cannot be equated with an individual’s immunity from SARS-CoV-2 infection.
Some tests may exhibit cross-reactivity with other coronaviruses, such as those that cause the common cold. This could result in false-positive test results. Some persons may not develop detectable antibodies after coronavirus infection. In others, it is possible that antibody levels could wane over time to undetectable levels. IgM and IgG antibodies are not present early in infection. Thus, serologic test results do not indicate with certainty the presence or absence of current or previous infection with SARS-CoV-2.
Recommendations for Use of Serologic Tests
Information that might impact serologic recommendations is rapidly evolving, particularly evidence of whether positive serologic tests indicate protective immunity or decreased transmissibility among those recently ill. These recommendations will be updated as new information becomes available.
Choice of test and testing strategy
- Serologic assays that have Emergency Use Authorization (EUA) are preferred for public health or clinical use since their test performance data have been reviewed by FDA.
- Serologic test results should be interpreted in the context of the expected predictive values, positive and negative.
- Positive predictive value should be optimized, particularly if results are returned to individuals, in the following ways:
- Assure a high positive predictive value (e.g., 95%) by choosing tests with sufficiently high specificity and testing persons or populations with a high pre-test probability of having antibodies (e.g., persons with a history of symptoms compatible with COVID-19 or who are exposed to areas or institutions experiencing outbreaks), OR
- If a high positive predictive value cannot be assured with a single test, use an orthogonal testing algorithm. See Table 1 for examples of using one or two tests in populations with various prevalences of SARS-CoV-2 antibodies.
- Currently, there is no substantive performance advantage of assays whether they test for IgG, IgM and IgG, or total antibody. Thus, immunoglobulin class should not determine the assay chosen in most circumstances. The detection of IgM antibodies may indicate a more recent infection, but the dynamics of the IgM antibody response are not well defined at present. Over time, it may be important to characterize and evaluate the performance of assays in samples that are IgM negative and IgG positive to ensure that assays remain fit for purpose in population studies as the pandemic progresses and more individuals are expected to have lower IgM levels.
- Serologic testing should not be used to determine immune status in individuals until the presence, durability, and duration of immunity is established.
- Serologic testing can be offered as a method to support diagnosis of acute COVID-19 illness for persons who present late.* For persons who present 9-14 days after illness onset, serologic testing can be offered in addition to recommended direct detection methods such as polymerase chain reaction. This will maximize sensitivity as the sensitivity of nucleic acid detection is decreasing and serologic testing is increasing during this time period.
- Serologic testing should be offered as a method to help establish a diagnosis when patients present with late complications of COVID-19 illness, such as multisystem inflammatory syndrome in children.
Recommendations for persons who test positive for anti-SARS-CoV-2 antibodies
- Although the presence of anti-SARS-CoV-2 antibodies when detected using a testing algorithm with high positive predictive value for the context of use likely indicates at least some degree of immunity, until the durability and duration of immunity is established, it cannot be assumed that individuals with truly positive antibody test results are protected from future infection.
- Asymptomatic persons who test positive by serologic testing and who are without recent history of a COVID-19 compatible illness have a low likelihood of active infection and should follow general recommendations to prevent infection with SARS-CoV-2 and otherwise continue with normal activities, including work.
- Persons who have had a COVID-19-compatible or confirmed illness should follow previous guidance regarding resumption of normal activities, including work.
- There should be no change in clinical practice or use of personal protective equipment (PPE) by health care workers and first responders who test positive for SARS-CoV-2 antibody.
Additional considerations on the use of serologic tests
- Serologic test results should not be used to make decisions about grouping persons residing in or being admitted to congregate settings, such as schools, dormitories, or correctional facilities.
- Serologic test results should not be used to make decisions about returning persons to the workplace.
- Until more information is available about the dynamics of IgA detection in serum, testing for IgA antibodies is not recommended.
* Detection of specific antibody in serum, plasma, or whole blood that indicates new or recent infection provides presumptive laboratory evidence of COVID-19 illness according to the Council of State and Territorial Epidemiologists (CSTE) interim case definition for COVID-19
Additional Resources
- American Medical Association. Serological Testing for SARS-CoV-2 Antibodies.external icon
- Infectious Diseases Society of America. IDSA COVID19 Antibody Testing Primer.pdf iconexternal icon
- Association of Public Health Laboratories and Council of State and Territorial Epidemiologists. Public Health Considerations: Serologic Testing for COVID-19. Version 1-May 7, 2020.pdf icon