In addition to today’s resource, please review:
Links To And Excerpts From “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation”
Posted on January 4, 2024 by Tom Wade MD
2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Circulation. 2024 Jan 2;149(1):e1-e156. doi: 10.1161/CIR.0000000000001193. Epub 2023 Nov 30.
Refere Kotalczyk, A., Lip, G. Y., & Calkins, H. (2021). The 2020 ESC Guidelines on the Diagnosis and Management of Atrial Fibrillation. Arrhythmia & Electrophysiology Review, 10(2), 65.
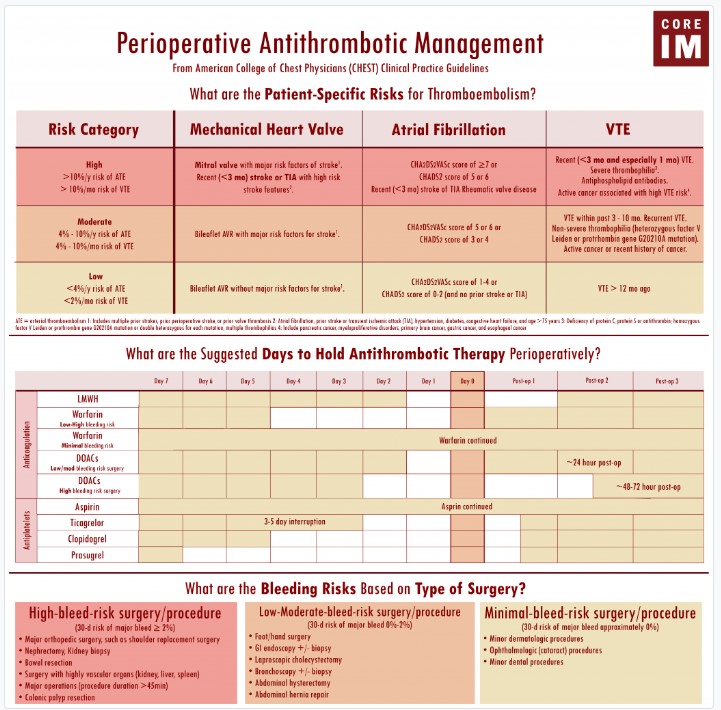
Douketis, J. D., Spyropoulos, A. C., Murad, M. H., Arcelus, J. I., Dager, W. E., Dunn, A. S., … & Moores, L. K. (2022). Perioperative management of antithrombotic therapy: an American College of Chest Physicians clinical practice guideline. Chest, 162(5), e207-e243.
Today, I review, link to, and excerpt from CoreIM‘s Atrial Fibrillation: Gray Matters Segment.*
*Posted: January 18, 2023
By: Dr. Nick Villano, Dr. Ali Trainor, Dr. Jason Matos, Dr. Greg Katz, Dr. Pooja Jagadish, Dr. Patrick Georgoff, Dr. Shreya P. Trivedi and Dr. Jason A. Freed
Graphic: Josie Levey
Audio: Daksh Bhatia
Peer Review: Mike Dunleavy, Susan Mcilvaine
All that follows is from the above resource.
Atrial Fibrillation: Gray Matters Segment
Show Notes
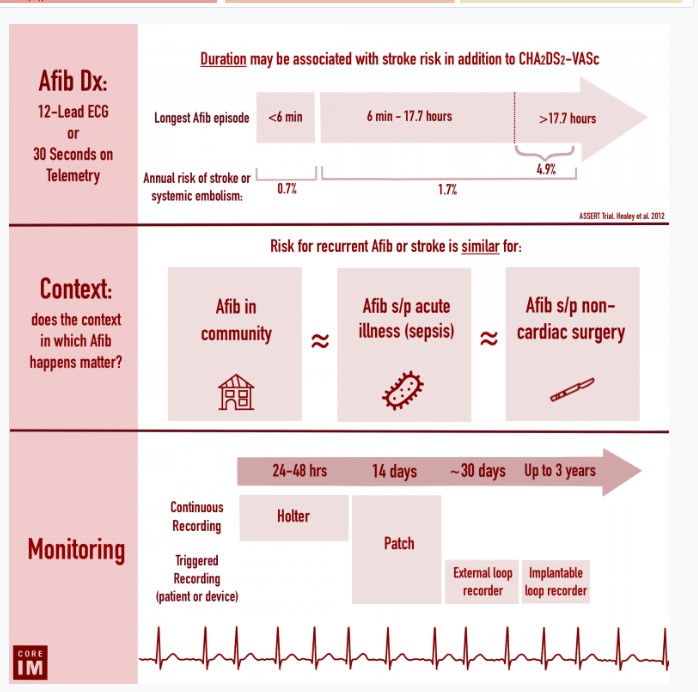
Diagnosis and Duration of Atrial Fibrillation
- The diagnosis of atrial fibrillation is made by a
- 12-lead electrocardiogram or
- At least 30 seconds of atrial fibrillation on telemetry
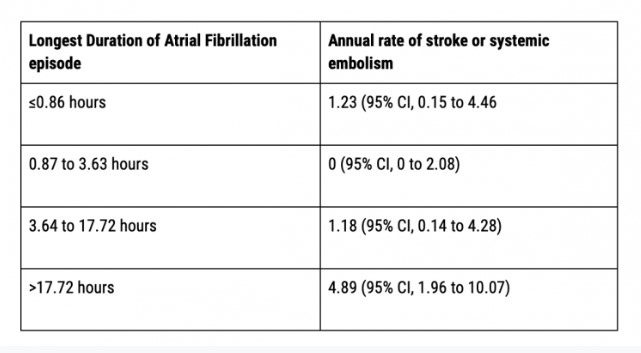
- Atrial fibrillation episodes of longer duration have been associated with an increased thrombotic risk in stable outpatients with long term cardiac monitors.
- This can be an additional objective factor in addition to the CHA2DS-VASc, however this is not currently reflected in any guidelines.
- Longest Duration of Atrial Fibrillation episode || Annual rate of stroke or systemic embolism (ASSERT Trial)
- The association between the frequency of atrial fibrillation episodes and thrombotic risk is less certain.
Atrial Fibrillation in Non-Cardiac Surgery or Acute Illness and Long and Short Term Outcomes
- Should we think of patients who develop afib after acute illness or non-cardiac surgery differently from those who have pre-existing atrial fibrillation in the community?
- Does putting patients with atrial fibrillation on anticoagulation after critical illness or non-cardiac surgery lower the risk of stroke?
- Non-cardiac surgery: There is evidence that the risk reduction of anticoagulation for thrombo-embolic stroke is similar between patients with atrial fibrillation diagnosed after non-cardiac surgery and in patients with pre-existing atrial fibrillation.
- Sepsis: There is a paucity of evidence (see sources 1 and 2) on the effect of anticoagulation for thrombo-embolic stroke for patients with atrial fibrillation diagnosed during sepsis.
- There are no clear guidelines on anticoagulation in these populations and practice patterns vary. The decision to initiate anticoagulation must be individualized based on a patient’s unique risk profile (using information such as CHA2DS2-VASc, AF burden, and bleeding risk).
- Why is atrial fibrillation after cardiac surgery thought of differently?
- Due to direct cardiac manipulation, atrial fibrillation following cardiac surgery is common and often resolves with cardiac healing. It is distinct from atrial fibrillation following non-cardiac surgery and warrants cardiac consultation to determine the optimal management.
- Is there an increased short-term risk of stroke for those newly diagnosed in the hospital with atrial fibrillation following sepsis or non-cardiac surgery?
- Non-cardiac surgery: Atrial fibrillation diagnosed after non-cardiac surgery is associated with an increased risk of 30 day or in-hospital ischemic stroke. However, it is important to consider the risk of bleeding post-operatively as well (see below).
- Sepsis: For those with newly diagnosed atrial fibrillation during severe sepsis, thromboembolic stroke risk is likely higher during the sepsis episode than for those with pre-existing or no atrial fibrillation. However, anticoagulation during sepsis did not reduce this risk and did increase clinically significant bleeding risk. This suggests it would be prudent to ensure a patient has fully recovered from their sepsis episode before considering anticoagulation.
Timing of Postoperative Anticoagulation
- Surgical considerations that may delay initiation of anticoagulation postoperatively include more extensive surgeries, trauma patients, and surgeries in confined areas where hematoma formation may be catastrophic (cranial, spinal, cardiac surgeries).
- The PAUSE trial supports resumption of DOACs 1 day after a low-bleeding risk procedure and 2-3 days after a higher risk intervention, provided hemostasis is achieved.
- The CHEST 2022 guidelines on perioperative anticoagulation management support the following in stable patients:
- Resuming warfarin 12-24 hours after surgery
- Resuming DOACs, enoxaparin, or bridging anticoagulation
- ~24 hours after low to moderate bleeding risk surgeries/procedures
- ~ 48-72 hours after high bleeding risk surgeries/procedures
fig4 when server ready
Utility of Post-Discharge Cardiac Monitoring in Newly Diagnosed Atrial Fibrillation Following Non-Cardiac Surgery or Acute Illness
- Continuous cardiac monitors record all telemetry information and are then sent in for analysis.
- Loop recorders (also known as event recorders) only save events triggered by a patient input or device detection.
- Some devices are hybrids, recording all data continuously while also isolating triggered events.
- Holter monitors are continuous monitors that often record for 24-72 hours. This can be useful to assess rate control in patients with permanent or frequently paroxysmal atrial fibrillation, but may not be enough time to detect infrequent episodes of atrial fibrillation.
- Patch monitors serve as both continuous and event monitors and generally record for 14 days. This can allow for improved detection of fibrillation episodes and help characterize the duration and frequency of these episodes.
- It is possible that 14 days may not be enough time and infrequent episodes of atrial fibrillation may still be missed by this monitor.
- Longer-term loop recorders may be required to detect more infrequent episodes of atrial fibrillation.
- These include external/event loop recorders (which may be worn for 30 days) and implantable loop recorders (which may be worn for up to 3 years).
- There are no guidelines regarding when to discontinue anticoagulation if no atrial fibrillation is detected. Longer monitoring and repeat discussions with the patient are often part of an ongoing risk-benefit discussion
- There is some evidence that wearable technology may be helpful in the detection of occult atrial fibrillation.
Discussing Atrial Fibrillation and Anticoagulation Risks and Benefits with Your Patient
- Anticoagulation adherence is low, about 50-60% in those on DOACs with newly diagnosed atrial fibrillation.
- Risk-benefit discussions regarding anticoagulation often involve new terms and multiple statistics. These factors, in addition to recent illness and a stressful hospitalization, often make it difficult for patients to fully engage in these discussions.
- Pausing to check in with the patient their understanding of atrial fibrillation can help tailor how to best present the nuances of atrial fibrillation and how much to present.
- Clear explanation with the patient of their post-discharge plan and communication with their outpatient providers can help ensure a safe transition of care.