Today, I review, link to, and excerpt from 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Circulation. 2024 Jan 2;149(1):e1-e156. doi: 10.1161/CIR.0000000000001193. Epub 2023 Nov 30.
All that follows is from the above resource.
Jump to
- Abstract
- Contents
- Top 10 Take-Home Messages
- PREAMBLE
- 1. Introduction
- 2. BACKGROUND AND PATHOPHYSIOLOGY
- 3. SHARED DECISION-MAKING (SDM) IN AF MANAGEMENT
- 4. CLINICAL EVALUATION
- 5. LIFESTYLE AND RISK FACTOR MODIFICATION (LRFM) FOR AF MANAGEMENT
- 6. PREVENTION OF THROMBOEMBOLISM
- 7. RATE CONTROL
- 8. RHYTHM CONTROL
- 9. MANAGEMENT OF PATIENTS WITH HF
- 10. AF AND SPECIFIC PATIENT GROUPS
- 11. FUTURE RESEARCH NEEDS
- PEER REVIEW COMMITTEE MEMBERS
- ACC/AHA JOINT COMMITTEE ON CLINICAL PRACTICE GUIDELINES
- PRESIDENTS AND STAFF
Abstract
AIM
The “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation” provides recommendations to guide clinicians in the treatment of patients with atrial fibrillation.
Top 10 Take-Home Messages
1. Stages of atrial fibrillation (AF): The previous classification of AF, which was based only on arrhythmia duration, although useful, tended to emphasize therapeutic interventions. The new proposed classification, using stages, recognizes AF as a disease continuum that requires a variety of strategies at the different stages, from prevention, lifestyle and risk factor modification, screening, and therapy. 2. AF risk factor modification and prevention: This guideline recognizes lifestyle and risk factor modification as a pillar of AF management to prevent onset, progression, and adverse outcomes. The guideline emphasizes risk factor management throughout the disease continuum and offers more prescriptive recommendations, accordingly, including management of obesity, weight loss, physical activity, smoking cessation, alcohol moderation, hypertension, and other comorbidities. 3. Flexibility in using clinical risk scores and expanding beyond CHA2DS2-VASc for prediction of stroke and systemic embolism: Recommendations for anticoagulation are now made based on yearly thromboembolic event risk using a validated clinical risk score, such as CHA2DS2-VASc. However, patients at an intermediate annual risk score who remain uncertain about the benefit of anticoagulation can benefit from consideration of other risk variables to help inform the decision, or the use of other clinical risk scores to improve prediction, facilitate shared decision making, and incorporate into the electronic medical record. 4. Consideration of stroke risk modifiers: Patients with AF at intermediate to low (<2%) annual risk of ischemic stroke can benefit from consideration of factors that might modify their risk of stroke, such as the characteristics of their AF (eg, burden), nonmodifiable risk factors (sex), and other dynamic or modifiable factors (blood pressure control) that may inform shared decision-making discussions. 5. Early rhythm control: With the emergence of new and consistent evidence, this guideline emphasizes the importance of early and continued management of patients with AF that should focus on maintaining sinus rhythm and minimizing AF burden. 6. Catheter ablation of AF receives a Class 1 indication as first-line therapy in selected patients: Recent randomized studies have demonstrated the superiority of catheter ablation over drug therapy for rhythm control in appropriately selected patients. In view of the most recent evidence, we upgraded the Class of Recommendation. 7. Catheter ablation of AF in appropriate patients with heart failure with reduced ejection fraction receives a Class 1 indication: Recent randomized studies have demonstrated the superiority of catheter ablation over drug therapy for rhythm control in patients with heart failure and reduced ejection failure. In view of the data, we upgraded the Class of Recommendation for this population of patients. 8. Recommendations have been updated for device-detected AF: In view of recent studies, more prescriptive recommendations are provided for patients with device-detected AF that consider the interaction between episode duration and the patient’s underlying risk for thromboembolism. This includes considerations for patients with AF detected via implantable devices and wearables. 9. Left atrial appendage occlusion devices receive higher level Class of Recommendation: In view of additional data on safety and efficacy of left atrial appendage occlusion devices, the Class of Recommendation has been upgraded to 2a compared with the 2019 AF Focused Update for use of these devices in patients with long-term contraindications to anticoagulation. 10. Recommendations are made for patients with AF identified during medical illness or surgery (precipitants): Emphasis is made on the risk of recurrent AF after AF is discovered during noncardiac illness or other precipitants, such as surgery. Guideline-Directed Management and Therapy
The term guideline-directed management and therapy (GDMT) encompasses clinical evaluation, diagnostic testing, and both pharmacological and procedural treatments. For these and all recommended drug treatment regimens, the reader should confirm dosage with product insert material and evaluate for contraindications and interactions. Recommendations are limited to drugs, devices, and treatments approved for clinical use in the United States.
Joshua A. Beckman, MD, MS, FAHA, FACC
Chair, ACC/AHA Joint Committee on Clinical Practice Guidelines
1.4. Scope of the Guideline
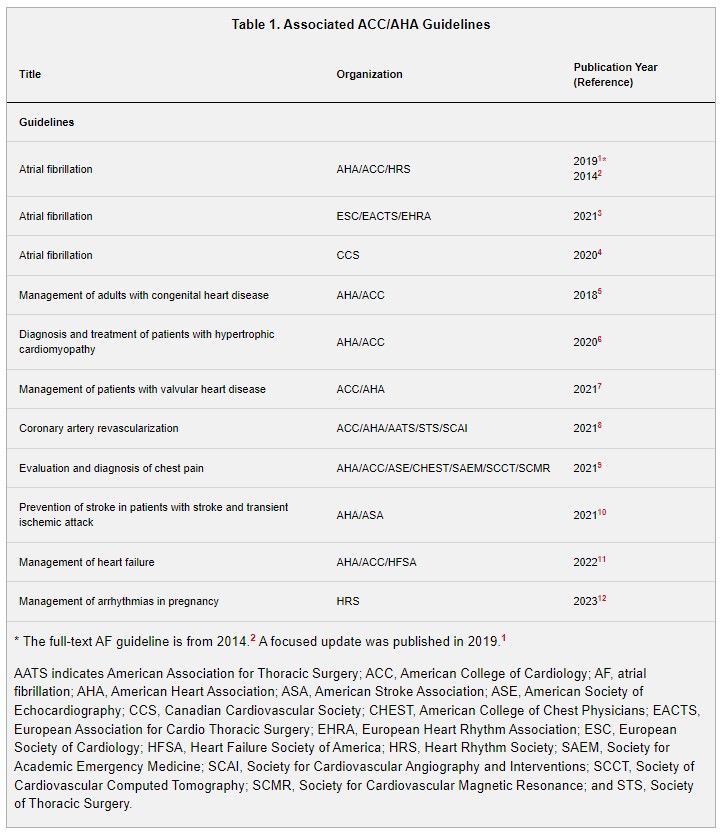
In developing the “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation” (2023 atrial fibrillation guideline), the writing committee reviewed previously published guidelines. Table 1 contains a list of these publications deemed pertinent to this writing effort and is intended for use as a resource, thus obviating the need to repeat existing guideline recommendations.
1.5. Class of Recommendations and Level of Evidence
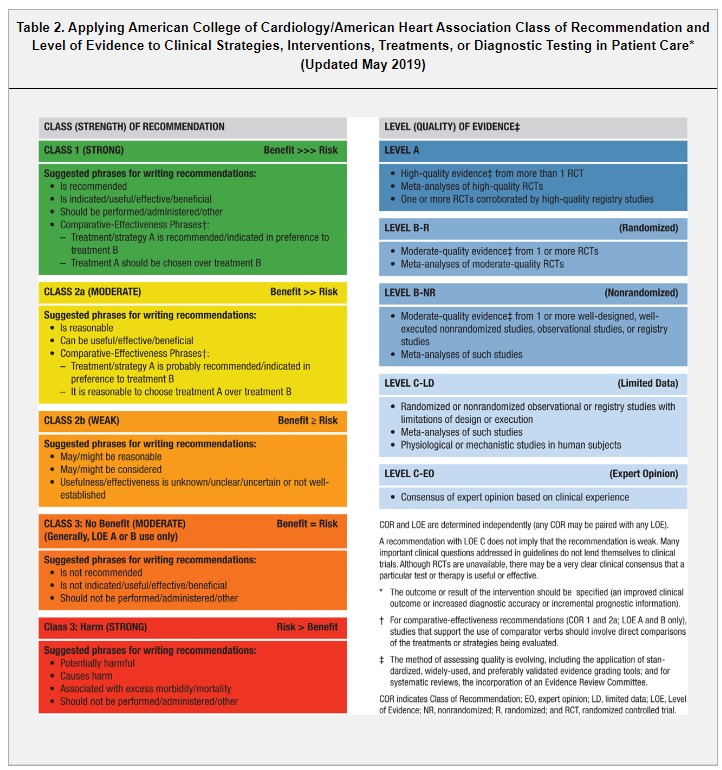
The Class of Recommendation (COR) indicates the strength of recommendation and encompasses the estimated magnitude and certainty of benefit in proportion to risk. The Level of Evidence (LOE) rates the quality of scientific evidence supporting the intervention on the basis of the type, quantity, and consistency of data from clinical trials and other sources (Table 2).
2. BACKGROUND AND PATHOPHYSIOLOGY
2.1. Epidemiology
Atrial fibrillation (AF) is the most sustained common arrhythmia, and its incidence and prevalence are increasing in the United States and globally (Figures 1 to 3).1,2 The increasing burden is multifactorial; causes include the aging of the population, rising tide of obesity, increasing detection, and increasing survival with AF and other forms of cardiovascular disease (CVD). The estimated global prevalence was 50 million in 2020.2,3 Although the prevalence of undiagnosed AF in the community is unknown, using back-calculation methodology, investigators have estimated that, in 2015, about 11% (591 000 cases) of the >5.6 million AF cases in the United States were undiagnosed.4
AF is associated with higher health care utilization and costs.2 Using US data from Optum (an administrative claims database for commercially insured [United Healthcare] patients in the United States), compared with patients without AF, patients with incident AF had an increased risk of inpatient visits and more cardiovascular-related emergency department visits (relative risk [RR], 2.41 [95% CI, 2.35–2.47]).5 AF is costly. Examining Optum data, individuals with AF have annual health care costs of $63 031, which is $27 896 more than individuals without AF.5 Investigators examining public and private health insurer data estimated that in US dollars in 2016, AF accounted for $28.4 billion (95% CI, $24.6 billion–$33.8 billion) in health care spending.6
2.1.1. Prevalence, Incidence, Morbidity, and Mortality
AF prevalence in the United States was estimated to be 5.2 million in 2010, with an expectation to rise to 12.1 million in 2030.1 Corresponding estimates for US incidence was 1.2 million cases in 2010, with an expectation to rise to 2.6 million cases in 2030.1 The rate of AF diagnosis varies by education, income,2 clinical,3,4 and genetic3 factors. Overall lifetime risk is about 30% to 40% in White individuals,2–4 about 20% in African American individuals,2 and about 15% in Chinese5 individuals.
AF is associated with a 1.5- to 2-fold increased risk of death6,7; studies suggest that the mortality risk may be higher in women than in men.6 In meta-analyses, AF is also associated with increased risk of multiple adverse outcomes, including a 2.4-fold risk of stroke,7 1.5-fold risk of cognitive impairment or dementia,8 1.5-fold risk of myocardial infarction (MI),9 2-fold risk of sudden cardiac death,10 5-fold risk of heart failure (HF),7 1.6-fold risk of chronic kidney disease (CKD),7 and 1.3-fold risk of peripheral artery disease (PAD).7 In Medicare beneficiaries, the most frequent outcome in the 5 years after AF diagnosis was death (19.5% at 1 year; 48.8% at 5 years)11; the next most common diagnosis was HF (13.7%), followed by new-onset stroke (7.1%), gastrointestinal hemorrhage (5.7%), and MI (3.9%).11
2.1.2. Risk Factors and Associated Heart Disease
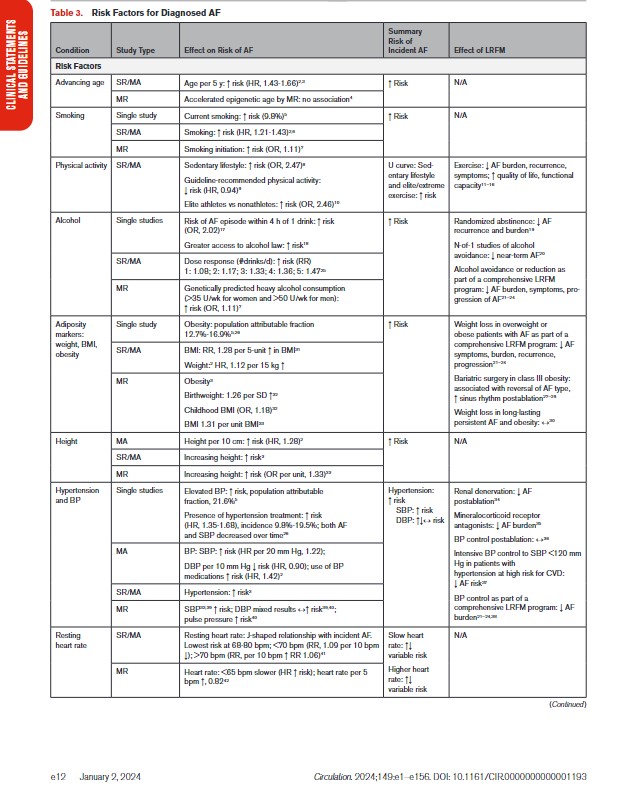
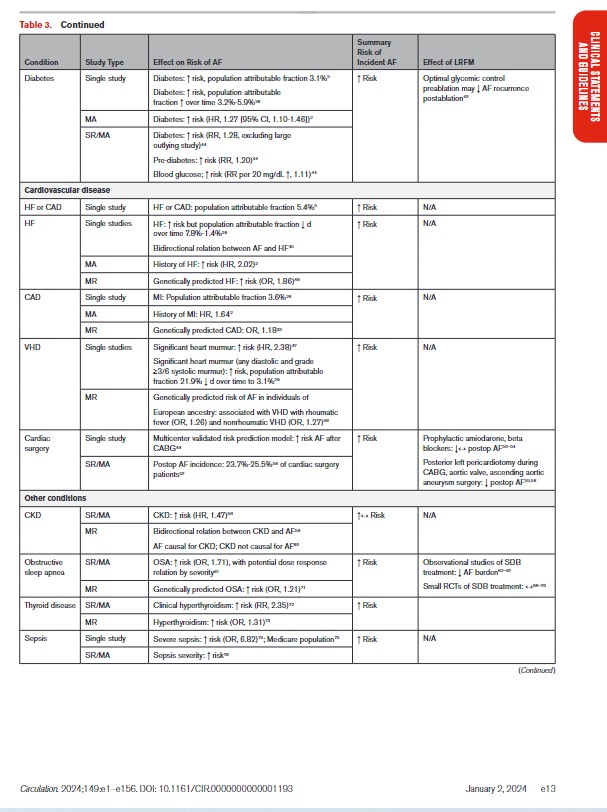
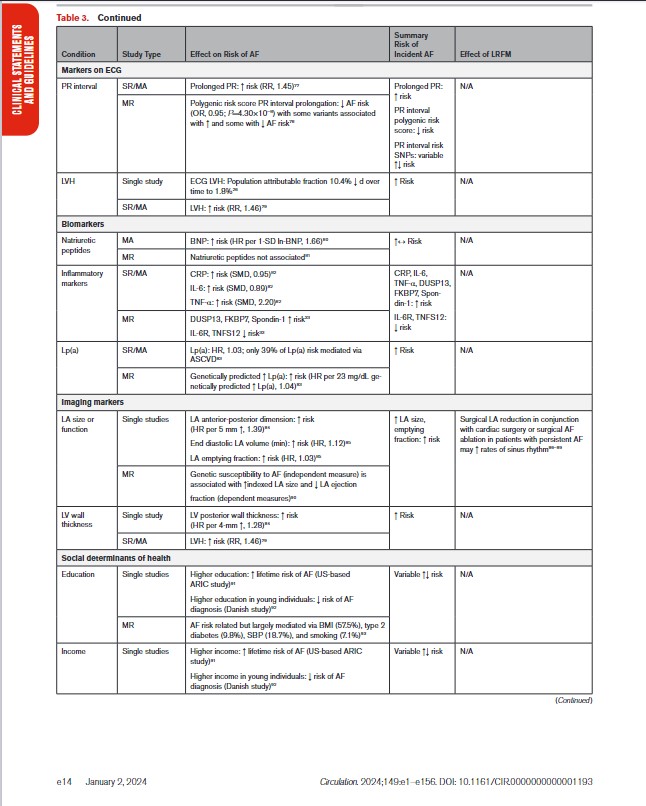
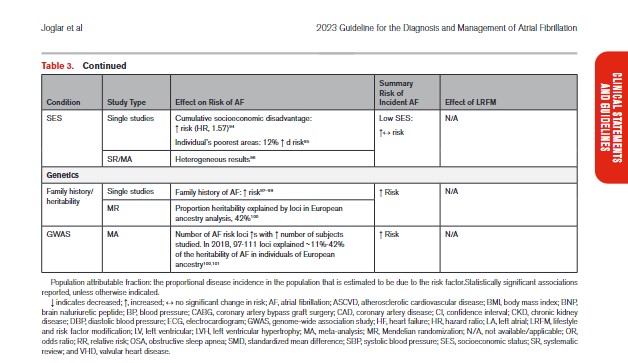
In Table 3, we present the evidence for the most widely reported and validated factors for AF from single studies, meta-analyses, and Mendelian randomization studies. Risk factors include demographic, anthropometric, and cardiovascular risk factors, CVD, noncardiac conditions, biomarkers (eg, electrocardiographic, imaging, circulating), and genetic markers.1 Models predicting risk of AF onset are presented in Section 4.1 (“Risk Stratification and Population Screening”). Most studies of AF risk factors and outcomes have been reported from high-income countries and in individuals of European ancestry.
2.2. Atrial Arrhythmia Classification and Definitions
2.2.1. AF Classification
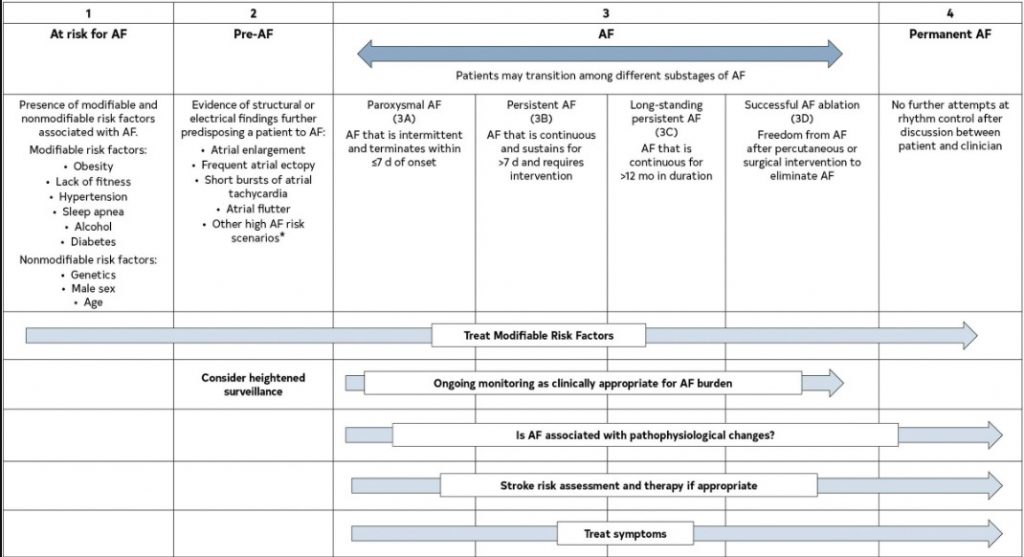
The previous classification of AF, which was based only on arrhythmia duration, although useful, tended to emphasize AF once it was diagnosed and focused mainly on therapeutic interventions. The new proposed classification using stages aims to correct the deficiencies of the previous classification by recognizing AF as a progressive disease that requires different strategies at the different stages, from prevention to screening, to rate and rhythm control therapies. The different stages better define AF as a progressive disease and highlight the need to address it at the earliest stages, especially emphasizing the importance of prevention, risk factor management, and timing for screening in those patients at the highest risk. The stages are not mutually exclusive (eg, risk factors should be managed through multiple stages) (Figure 4).
Figure 4. AF Stages: Evolution of Atrial Arrhythmia Progression. *Heart failure, valve disease, coronary artery disease, hypertrophic cardiomyopathy, neuromuscular disorders, thyroid disease. Original figure created by the 2023 Atrial Fibrillation Guideline Writing Committee. AF indicates atrial fibrillation.
AF is the most common arrhythmia in the world and accounts for significant morbidity and mortality. Over the past decade, evidence has consistently shown that the best treatment of atrial fibrillation requires multiple stakeholders committed to providing comprehensive patient-centered care. In addition, as emphasized in this guideline, AF should be thought of in a more holistic sense over an individual patient’s lifetime.
The foundation of optimal AF management is the treatment of risk factors and implementing lifestyle changes to decrease the likelihood of developing AF (Figure 5). Once AF develops, patient care should focus on assessing the risk of stroke and implementing any necessary treatment, continued optimization of all modifiable risk factors, and managing potential symptoms of AF, with an initial focus on evaluating and minimizing AF burden. However, as outlined in this guideline, access to all aspects of health care to all patients is necessary for any true improvement to be realized.
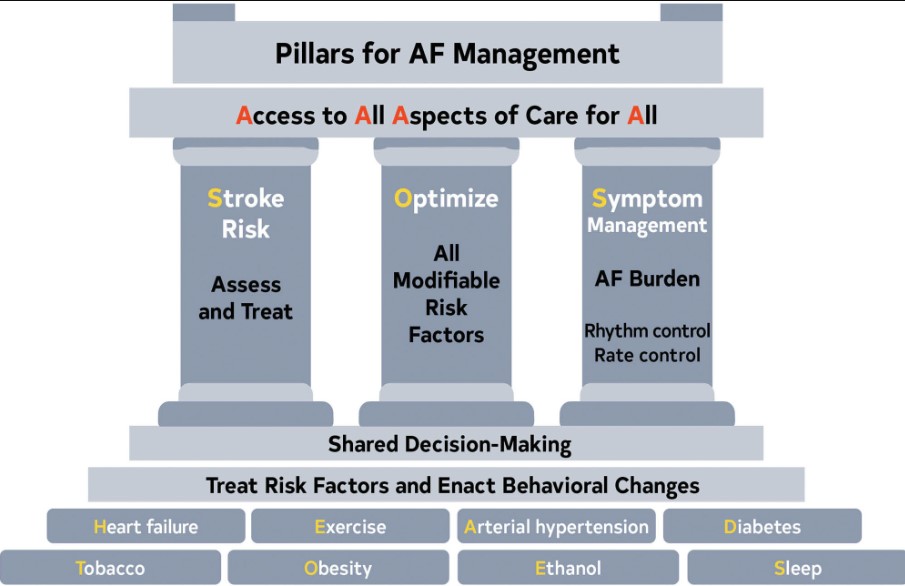
Figure 5. Pillars for AF Management. AF indicates atrial fibrillation.
When AF develops, holistic and optimal care of the patient at risk for AF, or who has developed AF, can be simply modeled using a building. The foundation of care is treatment of comorbidities and risk factors and implementing behavioral change in all individuals to decrease the likelihood of developing AF and reducing its burden (Screening for all risk factors from HEAD 2 TOES). Once AF develops, there are 3 important care processes that must be specifically addressed with all patients and aligned with their goals of therapy: Stroke risk assessment and treatment, if appropriate, Optimizing all modifiable risk factors, and Symptom management using rate- and rhythm-control strategies that consider AF burden in the context of an individual patient’s needs (SOS). The overarching principle for AF management is Access to All Aspects of care to All (4 As).
2.2.2. Associated Arrhythmias
Other atrial arrhythmias are often encountered in patients with AF.
Atrial Tachycardia (AT): It is a regular atrial rhythm at a constant rate of >100 beats per minute (bpm) with discrete P waves and atrial activation sequences originating outside of the sinus node.1 The mechanism can be automaticity, triggered activity, or a microreentry circuit. Focal ATs arise from a single discrete site within the left or right atrium, in contrast to macroreentrant atrial arrhythmias and AF, which involve multiple sites or larger circuits. In multifocal AT, the atrial activation sequence and P-wave morphology vary.
Atrial Flutter (AFL) and Macroreentrant AT: They occur in many of the same situations as AF. Typical AFL, also known as “typical AFL” or “cavotricuspid isthmus (CTI)-dependent AFL,”2 involves a macroreentrant circuit around the tricuspid annulus traversing the CTI on the right side of the heart (Figure 6). This is the arrhythmia associated with the classic electrocardiogram (ECG) finding of sawtooth flutter waves in the inferior leads when the circuit goes in the counterclockwise direction. The same circuit in the clockwise direction is called “reverse typical AFL.” If the flutter involves a different circuit than tricuspid valve/isthmus, then it is called “atypical” AFL, which is also known as “noncavotricuspid isthmus–dependent macroreentrant AT.”2 AFL was previously classified as either type I or type II. That terminology is no longer used.
2.3. Mechanisms and Pathophysiology
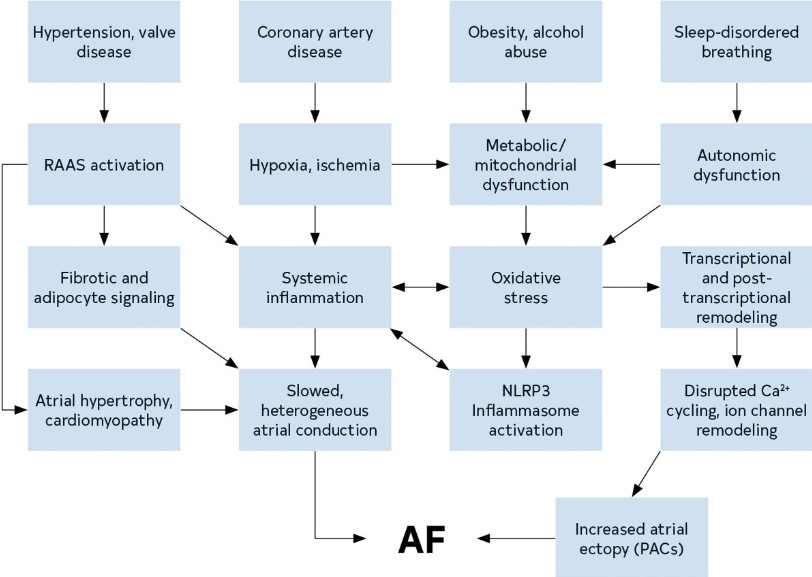
AF is a chaotic, rapid (300-500 bpm), and irregular atrial rhythm. Although normal rhythms are conducted through the atria in smooth waves initiated by the sinoatrial node, AF is the result of either electrophysiological abnormalities that underlie impulse generation and/or structural abnormalities of cellular connections that typically facilitate rapid and uniform impulse conduction. AF often stems from waves of electrical activity originating from ectopic action potentials most commonly generated in the pulmonary veins (PVs) of the left atrium (LA),1 or in response to reentrant activity promoted by heterogeneous conduction due to interstitial fibrosis.2 Atrial ectopy can generate runs of tachycardia, while persistent AF requires a substrate that is either sufficiently large or conduction that is sufficiently heterogeneous to enable reentrant activity to persist. The electrical abnormalities evident on the ECG during AF likely represent a shared phenotype of a condition with many distinct etiologies (genetic, environmental, and metabolic) (Figure 7).
Figure 7. Mechanisms and Pathways Leading to AF. The pathways that contribute to the development of AF create a substrate for reentry and provide triggers that can initiate arrhythmic activity. AF indicates atrial fibrillation; PAC, premature atrial contraction; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; and RAAS, renin-angiotensin-aldosterone system.
2.3.2.2. Persistence of AF
In general, persistence of AF reflects the substrate for AF. A suitable substrate for AF has a wavelength (wavelength = refractory period × conduction velocity) that is shorter than the dimensions of the tissue, with heterogenous conduction velocity and/or repolarization duration. Thus, an individual with large, fibrotic, and/or fatty atria is more likely to have persistent AF than one with a normal-sized atria with little interstitial fibrosis or adipose infiltration. Electrophysiologic remodeling is typically a response to persistent AF rather than the trigger.
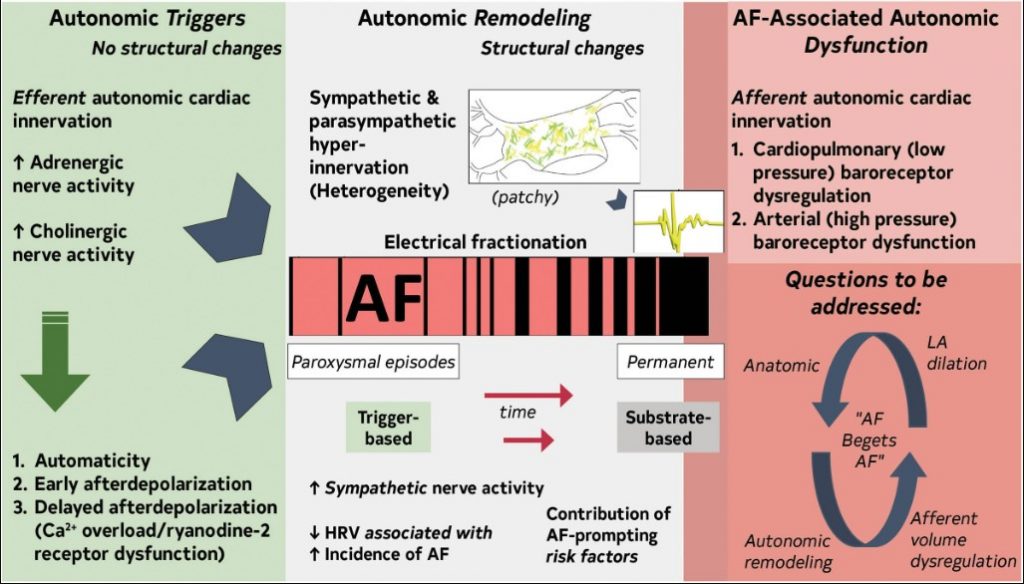
2.3.3. Role of the Autonomic Nervous System
The autonomic nervous system (ANS) has an important role as trigger and substrate (Figure 8).
Figure 8. Contemporary Summary of the Role of the ANS in AF.Original figure created by the 2023 Atrial Fibrillation Guideline Writing Committee. AF indicates atrial fibrillation; ANS, autonomic nervous system; HRV, heart rate variability; and LA, left atrium.
ANS triggers AF
The ANS as AF trigger is detailed in several reviews.1–6
ANS maintains AF
Atrial sympathetic and parasympathetic hyperinnervation and spatial heterogeneity, coupled with electrical fractionation and altered atrial electrophysiology, contribute to substrate.7–10 Modifiable AF risk factors promote ANS dysfunction.3
2.4. Genetics
Start here