In this post I link to and excerpt from Dr. Josh Farkas‘ Internet Book of Critical Care [Link is to the Table of Contents] Methemoglobinemia, October 17, 2019.
All that follows is from the above chapter.
CONTENTS
All that follows is from the above resource:
Physiology
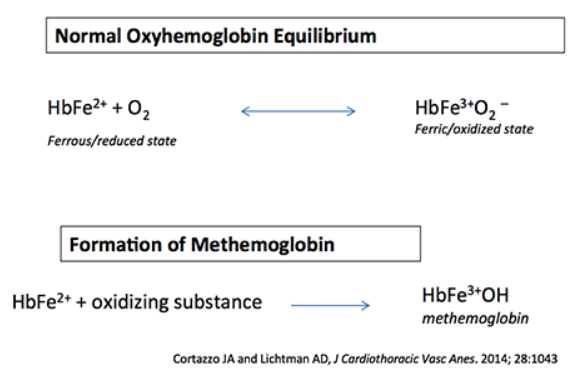
normal oxyhemoglobin equilibrium
- Oxygen binds to HbFe2+ (ferrous) state.
- During oxygen transport, this transiently forms the HbFe3+ (ferric) state.
- The ferrous (HbFe2+) form of iron is needed to bind oxygen.
formation of methemoglobin
- Various oxidizing substances convert the iron atom of hemoglobin into the Ferric(3+) state (forming methemoglobin). This is subsequently incapable of binding to oxygen (or transporting oxygen).
- Symptoms of methemoglobinemia result from inadequate oxygen transport.
Epidemiology
medication triggers
- Local anesthetics:
- Benzocaine (common cause, often used in endoscopic procedures).
- Prilocaine, Tetracaine, Lidocaine (rare).
- Nitrates:
- Nitroglycerine.
- Inhaled nitric oxide.
- Nitroprusside.
- Oral nitrates.
- Amyl nitrate.
- Antibiotics:
- Dapsone.
- Rifampin.
- Sulfonamides (e.g., sulfamethoxazole).
- Antimalarials (chloroquine, primaquine).
- Miscellaneous:
- Rasburicase (seems to associate with G6PD deficiency).
- Oncologic: cyclophosphamide, flutamide.
- Metoclopramide.
- Phenazopyridine (urinary analgesic agent).
- Street drug concocted using an oxidant drug ingredient.
environmental/occupational exposures
- Fertilizers, weed killers.
- Plastics.
- Dyes, paints, rubber.
clinical presentation
methemoglobin level & relationship to symptoms
- Below are general ranges.
- Severity of symptoms will also depend on the patient’s total hemoglobin concentration and cardiovascular reserves.
- Different sources disagree about exact numbers (none of this is strictly evidence-based).
- <2% is normal.
- 3-15%: Asymptomatic.
- Cyanosis can occur at levels above 5-10%.
- 20-30%: Moderate symptoms.
- Fatigue, tachypnea, dyspnea, tachycardia.
- Anxiety, dizziness, confusion.
- Nausea, vomiting.
- >40%: Severe symptoms can occur:
- Seizure, coma.
- Arrhythmia.
- Hyperlactatemia.
- Death.
#1 diagnostic clue: “refractory hypoxemia”
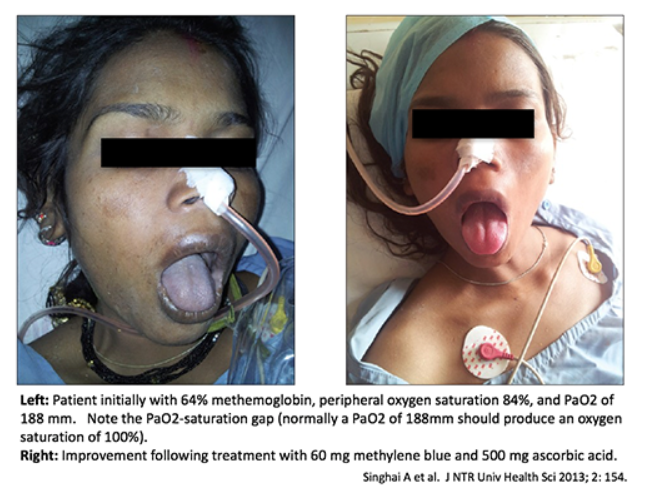
- Methemoglobinemia typically causes the pulse oximeter to report a saturation of ~82-86% (even if the PaO2 is very high).
- In a hospital, this will be interpreted as “refractory hypoxemia” (saturation in 80s despite 100% FiO2). Patients will be treated with high-dose supplemental oxygen and the ICU may be consulted.
#2 clue to diagnosis: cyanosis-saturation gap
- Methemoglobinemia often causes cyanosis or brown/grey discoloration of the skin. This may be a bit harder to diagnose in patients with darker skin (pay attention to the lips and tongue).
- As mentioned above, these patients will typically have a measured oxygen saturation of ~82-85%. An oxygen saturation in the 80s should not generally cause cyanosis (usually it must be substantially lower before cyanosis occurs). Thus, any time a patient has cyanosis with an oxygen saturation in the 80s, this suggests methemoglobinemia.
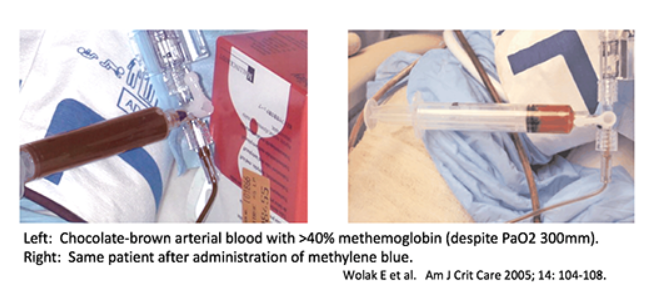
#3 clue to diagnosis: brown blood
- Methemoglobinemia may cause chocolate-brown discoloration of the blood.
- If blood is dropped on a piece of white gauze, it will remain brown as it dries (in contrast, normal deoxygenated blood will absorb oxygen and turn red).
diagnosis
There are a variety of approaches, which may depend on local resources and pre-test probability.
#1) ABG & PaO2-saturation gap
- Point-of-care ABG is a useful bedside test for methemoglobinemia.
- ABG will show the following:
- (1) PaO2 will be extremely high (typically >>100 mm), because the patient is being “treated” with high levels of supplemental oxygen. This immediately excludes true hypoxemia.
- (2) There is an obvious mis-match between the PaO2 (which is >>100 mm) versus the pulse oximetry (which is typically ~80-90% saturated). This is known as PaO2-saturation gap, and it’s indicative of some sort of hemoglobinopathy (most often methemoglobinemia).
- Some finer points will vary depending on how the ABG is analyzed:
- (1) Point-of-care ABG meters (e.g., iSTAT) will typically measure only the pO2, and then subsequently they calculate the predicted oxygen saturation based on that pO2 value. These meters will therefore report an oxygen saturation of ~99%.
- (2) If the ABG is sent to a central laboratory and evaluated with a formal blood gas analyzer, the analyzer may be able to detect methemoglobin directly (some hospitals will automatically test all ABGs and VBGs for carboxyhemoglobin and methemoglobin). This depends on the details of how the blood gas is analyzed in the lab.
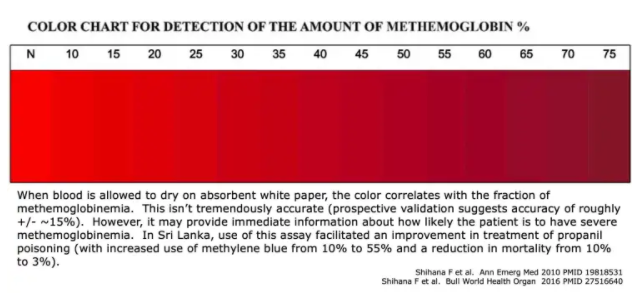
#2) bedside estimation of percent methemoglobin
- Blood may be placed on absorbent white paper.
- The color of the blood may be used to assess both the presence and severity of methemoglobinemia (above).
- This isn’t perfect, but it may provide some immediate information about the likelihood of severe methemoglobinemia.
- Either arterial or venous blood may be used (oxygen will be taken up from the atmosphere, thereby oxygenating the venous blood).
#3) formal measurement of methemoglobin level
- Any blood sample may be used (like carboxyhemoglobin, both venous and arterial blood samples will have the same level).
- Ordering will vary between different hospitals:
- Some hospitals will automatically measure levels of methemoglobin and carboxyhemoglobin in any blood gas analyzed in the laboratory (more sophisticated blood gas analyzers may measure this automatically).
- At hospitals where blood gas is performed at the bedside (using point-of-care analyzers), methemoglobin levels must be specifically ordered.
#4) consider empiric therapy with methylene blue
- It the clinical scenario is highly suggestive of methemoglobinemia and the patient is critically ill, it is reasonable to initiate therapy as soon as a blood specimen is drawn and sent to the lab.
- Methylene blue is generally safe (especially if the patient isn’t known to have G6PD deficiency).
- Administration of methylene blue can be both diagnostic and therapeutic.
treatment: general considerations
methylene blue
- Indications:
- Symptomatic methemoglobinemia.
- Methemoglobin level >30%.
- Possibly: Methemoglobin level >10-20%, in patients who are less likely to tolerate reduced oxygen delivery.(32193811)
- Contraindications:
- Dose:
- Give 1-2 mg/kg IV over 5 minutes.
- Clinical improvement should occur within minutes. However, methylene blue may cause transient drops in the measured oxygen saturation (due to its blue pigment). Cyanosis should resolve within an hour.
- Re-dosing:
- (a) Repeat dosing may be needed after 60 minutes if the initial dose is ineffective (e.g., persistent cyanosis). However, failure to respond to a dose of 2 mg/kg raises questions of G6PD deficiency. This can get a bit tricky, because methylene blue can interfere with the assay for methemoglobin (so avoid checking a methemoglobin level immediately after giving methylene blue).
- (b) Rebound methemoglobinemia can occur in 4-12 hours. Rebound may reflect the persistence of the causative agent (e.g., large volume ingestion).
- Toxicity from methylene blue:
- Doses >7 mg/kg may have significant side-effects (nausea, vomiting, confusion, dyspnea, tremulousness, diaphoresis).
- Doses >>7 mg/kg can paradoxically worsen methemoglobinemia.
additional treatment options
- Some benefit may result from riboflavin and intravenous vitamin C ~1.5-3 grams IV q6.(29592989, 27099330, 30944032)
- Dapsone-induced methemoglobinemia: High-dose cimetidine or ketoconazole may decrease metabolism into the (more toxic) dapsone hydroxylamine.(32193811)
- Exchange transfusion may be considered for patients failing to respond to other therapies.
treatment: G6PD deficient patient
basic physiology principles
- (1) G6PD deficiency causes a deficiency of NADPH. This NADPH deficiency leads to a deficiency of reduced glutathione, which subsequently causes hemolysis.
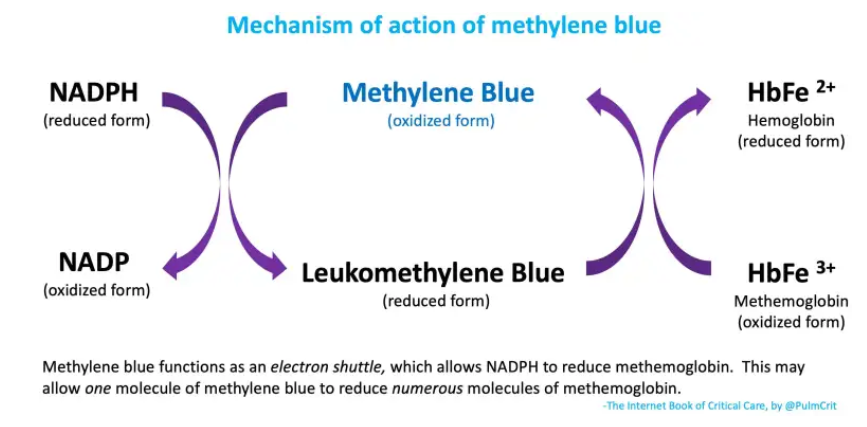
- (2) Methylene blue facilitates the use of NADPH to reduce methemoglobin back into normal hemoglobin (figure below). Methemoglobin isn’t actually consumed here – it acts as an electron shuttle which allows NADPH to reduce methemoglobin (figure below).
- This is great, because it gets rid of methemoglobin.
- The problem is that it consumes NADPH. In G6PD deficiency, this could theoretically exacerbate a deficiency of NADPH (promoting hemolysis).
- (3) Thus, there are two theoretical problems with using methylene blue in G6PD deficiency
- (a) Methylene blue may simply do nothing (it requires NADPH to work, and there isn’t much NADPH around).
- (b) Methylene blue may work, but in so doing it may drop NADPH levels further. This may cause hemolysis.
different types of G6PD deficiency
- This is further complicated by the existence of numerous different types of G6PD deficiency. The most common forms may be the following:
- (1) African-American males typically have a form of G6PD deficiency which most severely affects senescent, aging erythrocytes. So administration of methylene blue in these patients could work fine among younger erythrocytes.
- It’s possible that young erythrocytes (or possibly even endothelial cells?) could generate leucomethylene blue, which could then diffuse to older erythrocytes and treat their methemoglobinemia. Subsequently, methylene blue generated in older erythrocytes could diffuse back to the younger erythrocytes and so on – forming a persistent metabolic cycle between younger and older erythrocytes. This process might theoretically allow NADPH in the younger erythrocytes to reduce methemoglobin in the older erythrocytes!
- (2) G6PD deficiency among people of Mediterranean descent may affect erythrocytes of all ages. In these patients, methylene blue could be less effective and potentially more harmful.
evidentiary support
- Some case reports describe patients who received methylene blue and developed hemolysis. The problem here is that patients with G6PD deficiency who are under oxidative stress may develop hemolysis without any methylene blue!(22573495) It’s extremely difficult (or impossible) to tell if methylene blue plays a causative role in hemolysis among these patients.
- It’s dubious how much of an issue this is in practice. Methylene blue is commonly used without checking G6PD levels, and this doesn’t seem to cause frequent problems. Thus, the problems caused by G6PD deficiency may actually be overestimated.
reatment options in these patients:
- This is highly controversial, with no clear answer. Consult with a toxicologist to share the pain. Treatment options may include the following:
- (0) If the patient is doing OK, then careful observation might be the wisest approach.
- (1) The following treatments are safe and should arguably be used (but may not be the most effective):
- (a) IV vitamin C is a reducing agent which doesn’t require NADPH. Doses vary widely in the literature, but ~1.5-3 grams IV q6hr seems reasonable.(29592989, 27099330, 30944032)
- (b) Riboflavin (vitamin B2) can function as an electron shuttle, analogous to methylene blue. This seems to be an acceptable treatment here (although theoretically it could cause the same problem as methylene blue with regard to NADPH depletion).
- (2) Trial of methylene blue might be considered?
- Goldfrank’s Toxicology (11th Edition, Dennis Price) writes: “Judicious use of methylene blue is recommended in most patients with G6PD deficiency and symptomatic methemoglobinemia.”
- This might be more reasonable in African-American patients than Mediterranean patients?
- (3) Exchange transfusion (transfused erythrocytes will respond well to methylene blue).
PITFALLS
- Remember key clues to diagnosis:
- Refractory “hypoxemia” in patients with seemingly normal lungs.
- Saturation-PaO2 gap (patient’s saturation is in the 80s, but the PaO2 is normal or elevated).
- Saturation-cyanosis gap (patient develops cyanosis with only mild “hypoxemia” and a saturation in the 80s).
- Chocolate-colored blood.
- Exposures (particularly benzocaine spray to facilitate upper endoscopy or transesophageal echocardiography).
one-minute recap
Going further
- Must-read Tox & Hound post on methylene blue infusions here by Steve Curry.
- Methemoglobinemia (emDocs, Rana Biary)