Today, I review, link to, and excerpt from [The H2FPEF Score] A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Yogesh N V Reddy 1, Rickey E Carter 2, Masaru Obokata 1, Margaret M Redfield 1, Barry A Borlaug 1. [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
All that follows is from the above resource.
Abstract
Background
Diagnosis of heart failure with preserved ejection fraction (HFpEF) is challenging in euvolemic patients with dyspnea, and no evidence-based criteria are available. We sought to develop and then validate non-invasive diagnostic criteria that could be used to estimate the likelihood that HFpEF is present among patients with unexplained dyspnea in order to guide further testing.
Methods
Consecutive patients with unexplained dyspnea referred for invasive hemodynamic exercise testing were retrospectively evaluated. Diagnosis of HFpEF (case) or non-cardiac dyspnea (control) was ascertained by invasive hemodynamic exercise testing. Logistic regression was performed to evaluate the ability of clinical findings to discriminate cases from controls. A scoring system was developed and then validated in a separate test cohort.
Results
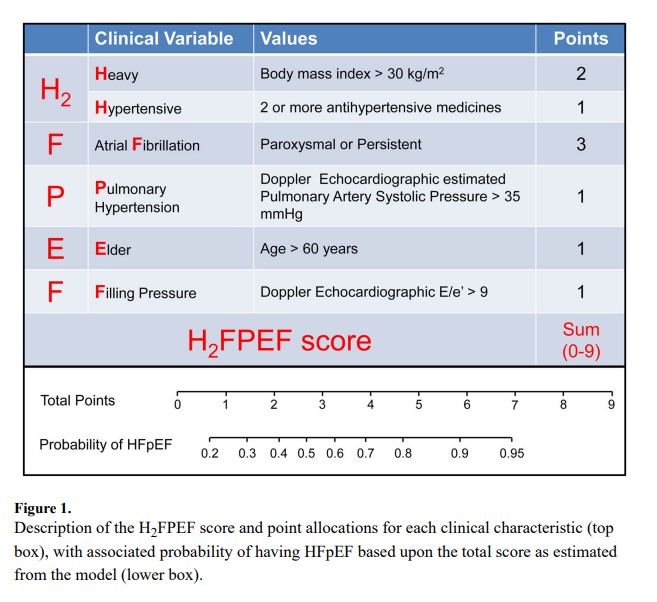
The derivation cohort included 414 consecutive patients (267 HFpEF and 147 controls, HFpEF prevalence 64%). The test cohort included 100 consecutive patients (61 HFpEF, prevalence 61%). Obesity, atrial fibrillation, age>60 years, treatment with 2 or more antihypertensives, echocardiographic E/e′ ratio>9 and echocardiographic pulmonary artery systolic pressure>35 mmHg were selected as the final set of predictive variables. A weighted score based on these six variables was used to create a composite score (H2FPEF score) ranging from 0–9. The odds of HFpEF doubled for each 1 unit score increase [OR 1.98 [1.74–2.30], p<0.0001], with an AUC of 0.841 (p<0.0001). The H2FPEF score was superior to a currently-used algorithm based upon expert consensus (increase in AUC of +0.169 [+0.120 to +0.217], p<0.0001). Performance in the independent test cohort was maintained [AUC 0.886, p<0.0001].
Conclusions
The H2FPEF score, which relies upon simple clinical characteristics and echocardiography, enables discrimination of HFpEF from non-cardiac causes of dyspnea, and can assist in determination of the need for further diagnostic testing in the evaluation of patients with unexplained exertional dyspnea.
Keywords: HFpEF, exercise testing, exercise catheterization
Introduction
Exertional dyspnea may be caused by cardiac and noncardiac disorders. Among the cardiovascular causes, heart failure with preserved ejection fraction (HFpEF) is an increasingly common etiology characterized by pathologic increases in cardiac filling pressures at rest or with exertion.1–6 Decompensated patients with HFpEF typically display overt congestion on physical examination and chest radiography, and in this setting the diagnosis is straightforward. However, compensated, euvolemic patients presenting with exertional dyspnea in the absence of overt clinical, radiographic or biomarker evidence of congestion present a greater diagnostic challenge.
The reference standard to diagnose HFpEF in these patients is by right heart catheterization followed by invasive exercise testing if resting intracardiac pressures are normal.7–10 Because of its invasive nature, technical complexity and cost, this test is impractical for routine evaluation, but is more logically reserved for situations where diagnosis remains uncertain after less invasive test results are equivocal.7 In order to make this determination, the probability of disease must first be estimated, allowing clinicians to decide whether disease is likely present or absent, or intermediate, where more definitive testing is required. Currently, there are no data available to guide this sort of Bayesian approach to the evaluation of unexplained dyspnea.
To fill this gap, we evaluated clinical data from consecutive patients where the diagnosis of HFpEF or a non-cardiac etiology of dyspnea was ascertained conclusively by invasive exercise testing in order to develop a scoring system that could be used in the diag
Discussion
Heart failure with preserved ejection fraction accounts for half of HF hospitalizations, and in hospitalized patients, overt congestion is typically obvious from physical examination, chest radiography and natriuretic peptide assays.1 However, in outpatients with exertional dyspnea, overt congestion is often absent at rest and the diagnosis may be challenging.7, 8 Right heart catheterization, with exercise if resting filling pressures are normal, is the gold standard for HFpEF diagnosis, but is not universally available, and non-invasive estimates of cardiac filling pressures lack sensitivity.1–8 In this study we derived and then validated a new score using clinical and echocardiographic variables that are widely available in clinical practice. In the derivation and test cohorts, and in sensitivity analyses restricted to community-based patients and those with early stage HFpEF, the H2FPEF score effectively discriminated patients with HFpEF from a comparator population of patients with exertional dyspnea that was not caused by heart failure, ascertained using the gold standard of invasive hemodynamic exercise testing. Inclusion of this control group was crucial to our study design, since it would not have otherwise been possible to judge the ability of clinical characteristics to estimate the likelihood of HFpEF without the ability to definitively identify or exclude disease based upon invasive criteria.
Diagnostic algorithms for HFpEF used in practice and for entry to clinical trials are based upon expert consensus opinion.4, 5 When these criteria have been prospectively evaluated, specificity was robust but sensitivity was poor.7 As such, HFpEF remains underdiagnosed in the community. In recent years, there has been increased utilization of invasive cardiopulmonary exercise testing to evaluate patients with exertional dyspnea, which is the gold standard to establish or refute the diagnosis of HFpEF.7–10 While this definitive approach has been shown to be cost-effective and safe,19 its uniform application is not practical for all diagnostic evaluations, given the enormous number of patients in the community presenting with exertional dyspnea.
By establishing the probability of disease, the H2FPEF score may be used to effectively rule out disease among patients with low scores (e.g. 0 or 1), establish the diagnosis with reasonably high confidence at higher scores (e.g. 6–9), and identify patients where additional testing is needed with intermediate scores (e.g. 2–5). Rather than forcing a probabilistic diagnosis (HFpEF) into binary categories (present or absent), this Bayesian approach provides a framework that can be used to determine whether there is sufficient confidence in the working diagnosis, or whether further evaluation is necessary based upon the identified probability of disease. This system could be readily applied for diagnostic purposes in clinical care as well as research settings to help refine enrollment criteria for clinical trials. Although the categorical H2HPEF score is easily calculated even at the bedside to rapidly estimate low or high probability of HFpEF, the more complex continual HFPEF calculator (Online Supplement) can also be used to provide a more precise estimate of the probability of HFpEF in an individual when required for clinical use, screening or research settings.
Selection of Final Model
In this analysis, we examined complementary modeling strategies that strove to balance parsimony, ease of calculation, and discriminatory capabilities. While we also considered more complex machine learning approaches, we finalized our models using multiple logistic regression analysis and the agnostic classification and regression tree analysis (CART). Many of the candidate variables for the models were highly collinear, so multiple sets of variables were often found to be equally discriminatory. The final model reflected a combination of variables selected a priori because of their central role in HFpEF pathogenesis (such as obesity and atrial fibrillation), as well as stepwise multivariable regression with systematic backwards elimination to only include variables that were independently predictive of HFpEF in combination. This yielded the components of our final H2FPEF score.
Sensitivity analyses using purely agnostic methods including an unbiased logistic model yielded nearly identical results, apart from the inclusion of right ventricular fractional area change in place of pulmonary artery systolic pressure (Supplemental Table 4). Because right ventricular fractional area change (a measure of right ventricular function) varies inversely with pulmonary artery pressure,20 it is not surprising that both measures can discriminate HFpEF from non-cardiac dyspnea. Since estimated pulmonary artery systolic pressure is a well-established marker of HFpEF21 and is more commonly measured in practice, we chose to include this in the final model rather than right ventricular fractional area change, which is not part of the routine clinical echocardiogram in many centers.
The lack of a particular variable in the final model (such as NT-proBNP) should not be interpreted as revealing a lack of association with HFpEF. Rather, what our data does suggest is that NT-proBNP may not add incremental information to clinical variables and echocardiography in diagnosing HFpEF among patients with unexplained dyspnea. This is in contrast to patients presenting with acute dyspnea that is present at rest, where the diagnostic performance of the natriuretic peptides are well established.22, 23 While discrimination of cases and controls was slightly improved using the classification and regression tree model and the continuous HFPEF score model, the differences were minor, and we propose that the simplicity of the H2FPEF score system outweighs this difference because it improves the feasibility of applying this approach in everyday practice. However, if precise estimation of an individual patient’s probability of underlying HFpEF is to be calculated, the use of the more complex continuous variable version of the HFPEF score from our online calculator can be applied.
Association of comorbidities with HFpEF
HFpEF is currently believed to be a systemic disorder driven in large part by comorbidities.2, 3 We observed that two comorbidities, obesity and atrial fibrillation, independently increase the probability that HFpEF is present. Severe hypertension identified by treatment with 2 or more antihypertensive drugs was another independent predictor. Diabetes is common in HFpEF, seen in 30–40%,24 but the presence of abnormal glucose tolerance did not add incremental diagnostic value beyond obesity alone, supporting the emerging evidence of the importance of obesity as an important cause of HFpEF.14
Limitations
NTproBNP data was missing at random in 24% of patients, due to the fact that some cardiologists did not obtain this laboratory during their evaluation. Therefore imputation was performed to account for the missing data, which may have affected the inclusion of NTproBNP in the final model. However, a sensitivity analysis yielded similar results in the 76% of patients that did have directly measured NTproBNP, increasing our confidence in the imputation derived values. This study was single center, limiting generalizability. There is referral bias in that all patients were referred for invasive testing, which may have inflated the prevalence of HFpEF. However, this analysis would not have been possible without the use of a gold standard assessment. Although this study was performed in a tertiary referral center, our practice also serves the local population and sensitivity analysis restricted to local patients revealed that the H2FPEF score performed similarly well in this subset (AUC 0.841), increasing confidence in generalizability of our results. While discrimination was maintained in our separate validation cohort, external validation was not performed and replication in other centers is necessary. Physical examination findings were not included in the models because there may be variability in examination skill and interpretation,25 and because overt congestion was absent in the patients included in this study, who were deemed to have indeterminate dyspnea after thorough evaluation by board certified cardiologists based upon history, physical examination and echocardiography. As such, the current results may not apply to patients with more frank evidence of tissue congestion, where testing beyond the history and physical examination may not be necessary to diagnose HFpEF. Assessment for lung disease was performed at the discretion of referring physicians and was not obtained in all patients. However, this reflects practice in the community, and the presence or absence of pulmonary disease is not relevant to the primary study goal of discriminating cardiac dyspnea (HFpEF) and non-cardiac dyspnea.
Conclusion
The H2FPEF score, which utilizes six clinical and echocardiographic characteristics that are universally obtained in the evaluation of patients with unexplained exertional dyspnea, enables robust discrimination of HFpEF from non-cardiac etiologies of dyspnea at low and high scores, while identifying patients at intermediate probability where additional testing is needed to refine diagnosis.