In this post, I link to and excerpt from Dr. Josh Farkas‘ Internet Book Of Critical Care [link is to the table of contents] chapter, Waveform Capnography in The Intubated Patient,
Note to myself and my readers: Go to the link above and review the entire chapter directly in the IBCC. I only make the excerpts because it helps me fix the chapter in my memory.
All that follows is from the above outstanding resource.
CONTENTS
- Rapid Reference
- Introduction: An emerging standard of care
- Physiology
- Clinical background
- Clinical utility
- Podcast
- Questions & discussion
- Pitfalls
rapid reference
introduction – an emerging standard of care
Waveform capnography is emerging as a standard monitoring tool to improve safety among intubated patients. Failure to use waveform capnography contributed to >70% of ICU-related airway deaths in the NAP4 audit. Capnography was pioneered in the operating room, but the safety implications for all critically ill patients are clear (the standard of safety monitoring in the ICU shouldn’t be lower than in the operating room). Capnography is increasingly recommended both to confirm endotracheal tube insertion and to subsequently monitor the patency and effectiveness of ventilation throughout the duration of intubation. Within the next decade, continuous waveform capnography will likely become a universal standard of care across all well-resourced intensive care units.
As the use of waveform capnography expands, we need to be thoughtful about integrating this into our practice. This involves several components:
- Paying attention to etCO2 values (e.g., noting them daily in reviews of the patient, along with other vital signs).
- Understanding changes in etCO2 within the context of other data (especially trends in minute ventilation).
- Understanding how to interpret etCO2 waveforms.
- Appreciating limitations of etCO2.
etCO2, PaCO2, and dead space
what is end-tidal CO2 (etCO2)?
- etCO2 is a measurement of the partial pressure of CO2 in gas expired at the end of exhalation (when exhaled gas will most closely resemble the alveolar CO2 concentration).
- Waveform capnography should be monitored in all intubated patients and displayed on the monitor (as above).
- Both the numeric value and the shape of the etCO2 tracing are important. If the etCO2 curve doesn’t reach a plateau, then the numeric value is less reliable.
understanding dead space
- Dead space refers to inhaled gas that doesn’t participate in carbon dioxide clearance. Three general sources of dead space are:
- Anatomic dead space (e.g., trachea and large bronchi) – with each breath gas enters these areas, but doesn’t participate in gas exchange.
- Instrument dead space – for intubated patients, this includes any tubing interposed between the patient’s airway and the site of fresh gas insufflation.
- Alveolar dead space – dysfunctional alveoli which receive ventilation but don’t exchange CO2 well (or at all).
- Anatomic and instrument dead space are generally fixed. However, if the patient is ventilated with small tidal volumes, then a greater fraction of each breath will be wasted on dead space ventilation. Consequently, a strategy of high-frequency, low-tidal volume breaths will tend to achieve less CO2 clearance for any specific total minute ventilation.
- Alveolar dead space may be increased in most types of lung disease (reflecting dysfunction at the alveolar, vascular, or airway level). For example, increased dead space is seen in pulmonary embolism, in pneumonia or other parenchymal lung diseases such as aspiration, or in obstructive lung diseases such as asthma. The amount of alveolar dead space may change over time, as lung disease improves or deteriorates.
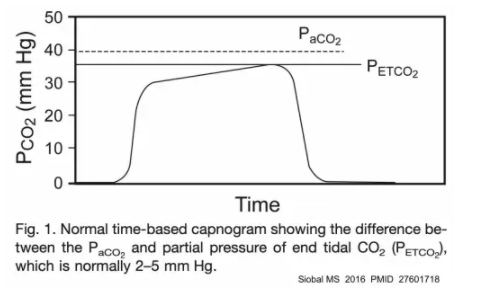
the relationship between etCO2 and arterial CO2 (PaCO2)
- The best way to conceptualize this is to imagine gas flowing from a high-functioning alveolus into the ventilator.
- #1) Within the high-functioning alveolus, the CO2 pressure will be equal to the arterial CO2 pressure.
- #2) As gas flows from this alveolus out of the lung, it will be diluted by dead space gas that will have a lower CO2 concentration (since this dead space gas doesn’t absorb CO2 from the blood).
- #3) By the time gas reaches the endotracheal tube, the end-tidal CO2 concentration will be lower than the arterial CO2 tension.
- This leads to two foundational principles of etCO2:
- (1) Arterial CO2 should be higher than etCO2. Extraordinarily rarely, etCO2 can be slightly higher than arterial CO2 in pregnant patients with otherwise normal lungs.
For the purpose of everyday critical care practice, it’s reasonable to assume that the PaCO2 is above the etCO2.
- (2) The gap between the etCO2 and the PaCO2 is a reflection of the amount of dead space. Any increase in dead space (e.g., due to severely injured lungs), will widen the gap. In patients with normal lungs, the gap is typically ~3-10 mm. For patients with severe lung disease, the gap can be much greater.
Upload fig4 when server is ready.
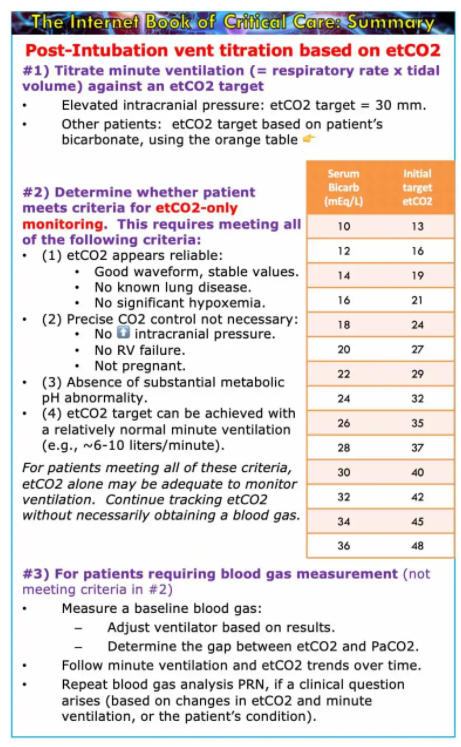
etCO2 to guide initial ventilator settings
Immediately after intubation, etCO2 can help adjust the ventilator settings. Even if you are going to check a blood gas, it still makes sense to begin by adjusting the ventilator based on etCO2 (and subsequently checking your work using a blood gas). Immediately adjusting the ventilator based on etCO2 may accelerate achieving a target pH and thereby avoid the requirement for multiple blood gas measurements.
for a patient with critical neurologic disease
- In this context it’s often desirable to target a low-normal PaCO2 (to avoid cerebral vasodilation or vasoconstriction).
- Following intubation, adjust the ventilator to target an etCO2 of ~30 mm. This will generally result in a PaCO2 within the normal range (35-45 mm). Then check a blood gas, to ensure that the pCO2 is actually within a safe range.
for patients without critical neurologic disease
- For most patients, targeting a pH of 7.2-7.5 may be reasonable (discussed further above).
- If we know the patient’s baseline bicarbonate value, then we can use this to establish an initial etCO2 target (table below).
- The etCO2 target is determined by calculating the PaCO2 which would give the patient a pH of 7.5.
- In reality, the PaCO2 will be greater than the etCO2. This will place the patient’s pH into a safe range (7.2-7.5). For most patients, the gap between etCO2 and PaCO2 will be ~5-10 mm, which will leave them at the higher end of this range (e.g., a pH of ~7.4). Patients with lung disease and a larger etCO2-PaCO2 gap, may have a somewhat higher PaCO2 and a thus a lower pH (e.g., a pH of ~7.3) – but their pH will still likely lie within a safe range.
- Targeting an etCO2 immediately, based on the patient’s baseline bicarbonate, should rapidly bring the patient to a safe pH range (thereby avoiding common mistakes, such as post-intubation alkalemia in a patient with chronic hypoventilation and elevated bicarbonate). If necessary, further fine-tuning may be based on the results of blood gas analysis.
Post fig 5 when server is ready.
Start here.