The goal of parents and physicians is the recognition and treatment of seriously ill pediatric patients at the earliest opportunity before the newborn, infant, or child is obviously in trouble [and later recognition of serious illness might be too late].
The accurate measurement of pediatric vital signs is critical to this task.
And obtaining an accurate pediatric respiratory rate can be as difficult in a U.S. primary care office as obtaining one in a resource limited area.
Accurate determination of pediatric heart rate is also of critical importance and is also difficult to accurately measure in pediatric care.
Pediatric tachypnea can be the first sign of a serious pediatric illness in a pediatric patient who initially look well.
We don’t want to assume that the pediatric tachypnea is just due to anxiety. Rather, we will need to carefully to rule out more serious causes of the tachypnea (with careful history and physical exam including head-t0-toe skin exam and physical exam [sometimes with careful capillary refill, non-invasive blood pressure evaluation, pulse oximetry, capnography, and and accurate heart rate determination]).
Examples include pediatric tachypnea due to diabetic ketoacidosis, sepsis, pneumonia, acute abdomen, or any other cause of a significant metabolic acidosis (as, for example, the first sign of significant hypovolemia from dehydration or bleeding).
The pediatric tachypnea that occurs in metabolic acidosis is the result of the body’s attempt at respiratory compensation (a result of the compensatory respiratory alkalosis). And a component of the pediatric tachypnea is also caused by the activation of the sympathetic nervous system that occurs with serious illness.
Massimo has just received FDA clearance for a new technology that will allow it to integrate respiratory rate determination via photoplethysmography into its MightySat Rx finger pulse oximeter for adults.
Integrated pediatric monitors are available that can measure oxygen saturation, heart rate, respiratory rate for the primary care office. They cost approximately $2000.
The above monitors can significantly decrease the risk of missing abnormal pediatric vital signs.
In this post I link to and review excerpts from A Systematic Review of Tools to Measure Respiratory Rate in Order to Identify Childhood Pneumonia [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Am J Respir Crit Care Med. 2018 May 1;197(9):1116-1127.
Abstract:
Pneumonia is the leading infectious cause of death in children worldwide, with most deaths occurring in developing countries. Measuring respiratory rate is critical to the World Health Organization’s guidelines for diagnosing childhood pneumonia in low-resource settings, yet it is difficult to accurately measure. We conducted a systematic review to landscape existing respiratory rate measurement technologies. We searched PubMed, Embase, and Compendex for studies published through September 2017 assessing the accuracy of respiratory rate measurement technologies in children. We identified 16 studies: 2 describing manual devices and 14 describing automated devices. Although both studies describing manual devices took place in low-resource settings, all studies describing automated devices were conducted in well-resourced settings. Direct comparison between studies was complicated by small sample size, absence of a consistent reference standard, and variations in comparison methodology. There is an urgent need for affordable and appropriate innovations that can reliably measure a child’s respiratory rate in low-resource settings. Accelerating development or scale-up of these technologies could have the potential to advance childhood pneumonia diagnosis worldwide.
Excerpts:
Pneumonia remains the leading infectious cause of death among children younger than 5 years of age. In 2015, pneumonia accounted for 16% of child deaths globally (1). Although childhood pneumonia deaths can be prevented with simple interventions and appropriate treatment, pneumonia often goes undiagnosed and untreated in the community until the child is severely ill (2, 3). In low-resource settings, pneumonia is diagnosed using the World Health Organization Integrated Management of Childhood Illness and Integrated Community Case Management (iCCM) guidelines, which rely on the appreciation of subjective clinical signs and symptoms.
An important component of the Integrated Management of Childhood Illness and iCCM criteria is the accurate classification of fast breathing, defined as
- 60 or more breaths per minute in infants younger than 2 months,
- 50 or more breaths per minute in infants aged 2 to 11 months, and
- 40 or more breaths per minute in children aged 12 to 59 months (4).
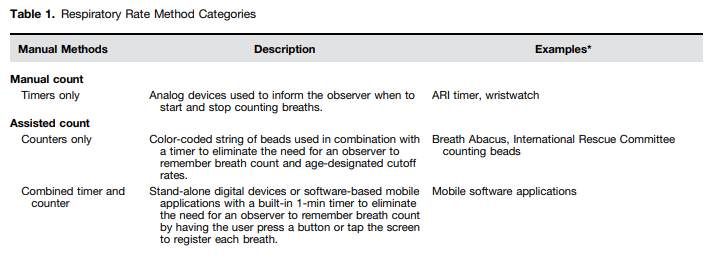
In low-resource settings, counting the number of breaths typically is performed manually with the aid of watches, timers, and counting beads (5, 6).
Accurate assessment of RR is critical in low-resource settings where other diagnostic tools, such as pulse oximetry or chest radiography, are typically not available and pneumonia is diagnosed based on the child’s clinical signs alone. Given the high burden and mortality of childhood pneumonia [and in resource rich nations, the need to recognize the subtle early signs of potentially serious pediatric illness], there is growing demand for better ways to measure RR accurately and reliably.
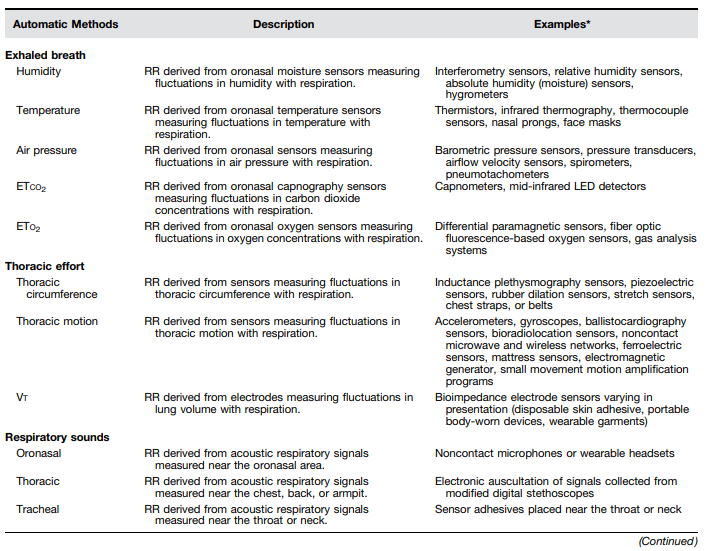
Although there are numerous existing and potential approaches for measuring RR (Table 1), it is important to assess whether these have been rigorously evaluated in a way that facilitates comparisons of accuracy and performance. This systematic review provides an overview of the RR measurement tools that have undergone a clinical evaluation of accuracy against a reference standard among spontaneously breathing children younger than 5 years of age. Some of the results of these studies have been previously reported in the form of an abstract (8).
Results:
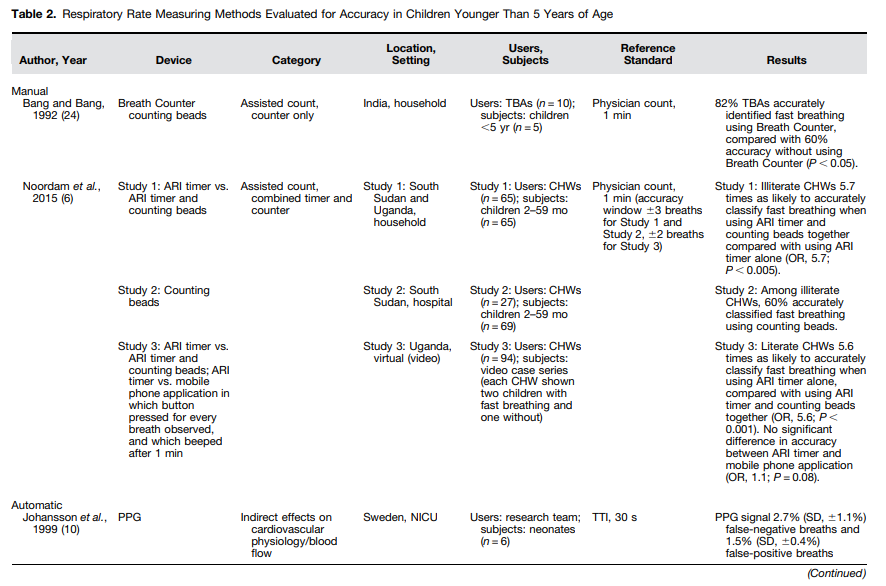
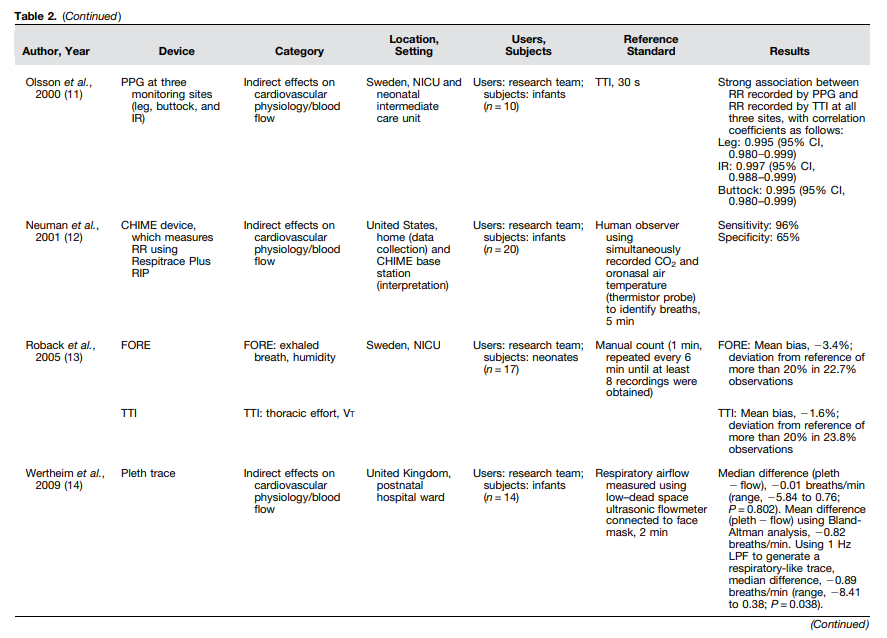
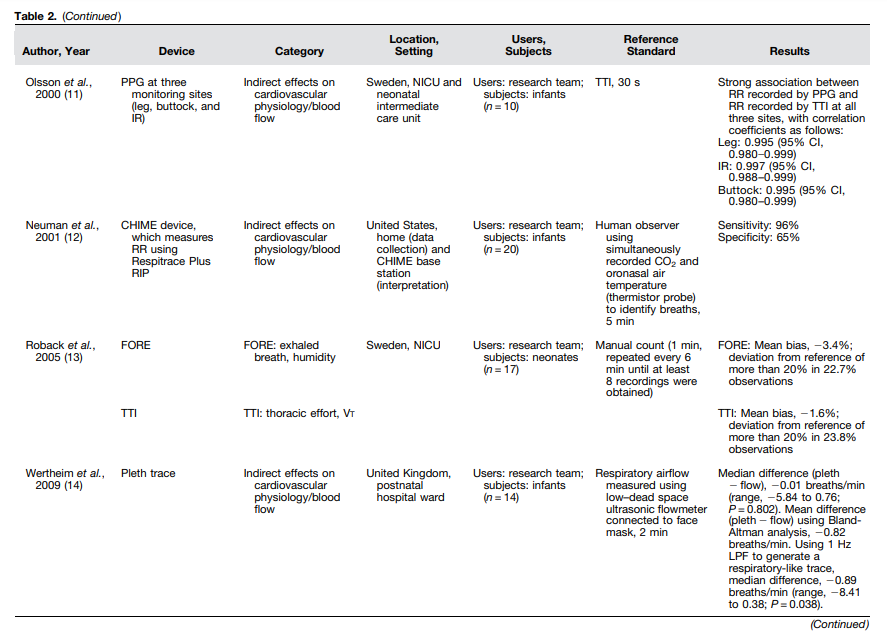
Studies Included through Systematic ReviewFrom a total of 7,669 unique citations, 89 publications were identified as sufficiently relevant for full text review, and 16 were ultimately included (Figure 1). Fourteen of those papers reported on automated devices, and two reported on manual devices (Table 2). All included studies on automated devices reported data from well-resourced settings, including the United Kingdom (n = 4), Sweden (n = 3), the Netherlands (n = 3), the United States (n = 1), Israel (n = 1), Switzerland (n = 1), and Australia (n = 1) (10–23). Both papers on manual devices reported data from low-resource settings, with one conducted in India and the other consolidating evidence from studies in Ghana, South Sudan, and Uganda (Table 2) (6, 24).
Table 2:
Manual DevicesThe two publications describing the accuracy of fast breathing assessment using manual devices included primarily low literacy, community-based, frontline providers in low-resource settings and used a reference standard of clinician count over 1 minute (Table 2). In a study conducted in India in the early 1990s, the accuracy of fast breathing assessment by 10 traditional birth attendants was higher when assisted by age-specific color-coded counting beads in comparison to no device (82 vs. 60%) (24). In more recent years, studies assessing the relative benefit of counting beads in conjunction with an acute respiratory infection timer have been conducted to inform iCCM guidelines on pneumonia (6).
Formative research in Ghana suggested that color-coded beads could facilitate classification accuracy by assisting community health workers (CHWs) in identifying fast breathing by age without the need to remember age-specific cutoff rates. Pooled data from studies among primarily illiterate CHWs in South Sudan and Uganda indicate a 41% absolute increase in the accuracy of fast breathing classification when assisted by counting beads in conjunction with a timer compared with an acute respiratory infection timer alone (odds ratio [OR], 5.7; P < 0.005) (Table 2).
In South Sudan, the use of counting beads enabled 60% of illiterate CHWs to accurately classify fast breathing. In Uganda, findings differed based on the literacy level of the CHWs. Among illiterate CHWs, the ability to classify fast breathing increased from 37% using the timer alone to 73% using the timer and counting beads combined (OR, 4.4; P < 0.005). However, literate CHWs were 5.6 times as likely to report a breath count within plus or minus two breaths of the reference standard when assisted with a timer alone compared with a timer in conjunction with counting beads (OR, 5.6; P < 0.001). A mobile phone application was also assessed in this study, and no significant difference in accuracy was noted between the timer and the mobile phone application (OR, 1.1; P = 0.08) (6).
Among literate CHWs using any method, breath count was typically more accurate at slower breathing rates rather than faster rates, and CHWs tended to overestimate RR in the slow-rate scenario and underestimate RR in the fast-rate scenario.
Automated DevicesWe identified 14 studies assessing the accuracy of automated breath counters (Table 2). One study assessed multiple devices, for a total of 15 technologies assessed. Automated breath counters fell into four categories: indirect effects on cardiovascular physiology/blood flow (n = 8); thoracic effort, Vt (n = 3); thoracic effort, thoracic motion (n = 3); and exhaled breath humidity (n = 1) (Table 2).
Discussion
Given the immense burden of childhood pneumonia and the fact that RR is the primary method for diagnosing pneumonia in low-resource settings, it is critical to understand the landscape of RR measurement technologies. This systematic review identified 3 manual and 15 automated RR counting devices evaluated for accuracy among spontaneously breathing children younger than 5 years of age. Although automated technologies were divided a priori into 4 categories and 16 subcategories (Table 1), only 4 of the subcategories were identified in this review: PPG (n = 8), Vt (n = 3), thoracic motion (n = 3), and exhaled breath humidity (n = 1) (Table 2). Although devices in other categories may be in the development or evaluation stage, these four categories represent devices whose accuracy has been assessed against a reference standard. No devices in the respiratory sounds category were identified in this review. Acoustically derived respiratory devices may be subject to signal artifact, leading to difficulties in obtaining an accurate count. On the basis of the devices identified in this review, promising RR technologies include noncontact devices, those that can detect changes in motion or color among children of a range of ages and skin tones, and those that may be integrated into an existing device like a pulse oximeter or a multiuse device such as a tablet or smart phone.
A rigorous evaluation of accuracy is necessary to validate any technology before widespread use as a diagnostic tool. Although all studies in this review included an assessment of accuracy, there was wide variation in reference standard selection and statistical methods of comparison. This variation precluded the ability to compare devices head-to-head and complicated attempts to develop standard criteria or a cutoff for determining accuracy. . . . Consensus regarding an appropriate reference standard would improve the generalizability of future RR device evaluations.
Although manual breath count was the most common reference standard in this review, it is known to have issues with accuracy and reliability (13, 18). Challenges to manual count include distraction, an agitated or crying child, and mistaking nonrespiratory movements and sounds as breaths (13, 18, 25).
The appropriate methods and the challenges involved in measuring agreement between a clinical standard and a new device have previously been described in detail (26, 27).
Although all of the manual devices assessed were evaluated in low-resource settings, all of the automated devices were tested in well-resourced settings, often in tightly controlled environments. In the absence of validation in low-resource settings, it is difficult to assess the strengths and weaknesses of the devices included in this review. However, low-cost devices with multiple uses (including the CHIME monitor and those that assess RR using a pulse oximeter) may have an advantage over single-purpose devices, because they would allow providers to assess multiple vital signs with a single device, potentially reducing the complexity and training involved in incorporating a new technology into clinical care.
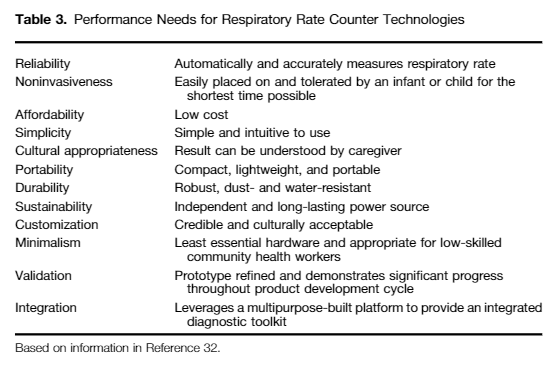
Characteristics of an RR counter most promising for advancement include portability, durability, and low cost (Table 3). The RR device should be simple to use and should provide the result in a way that a CHW or the child’s caregiver can understand (31, 32).
In performing a landscape analysis of available RR technologies, it is also important to address the value of RR as a diagnostic tool for pneumonia. Many factors can influence RR, including temperature, agitation, and whether the child is awake. A 2017 systematic review assessing the accuracy of clinical symptoms in identifying children with radiographic pneumonia found that among children with cough, fever, or both, tachypnea did not increase the likelihood of radiographic pneumonia (33). Although tachypnea may be a tool in the diagnosis of pneumonia, tachypnea should be assessed in tandem with other clinical signs and symptoms (such as work of breathing)* and should not act as a stand-alone diagnostic criterion.
*along with accurate assessment of the heart rate, temperature, and capillary refill.
ConclusionsAssessment of RR is integral to the pneumonia diagnostic pathway in low-resource settings. Accelerating the development of innovations and spurring the adaptation of current devices could significantly improve the process of pneumonia diagnosis.