In addition to today’s resource, please review:
- Evaluation of Venous Congestion Using Beside Ultrasonography by the Nephrology Consultant: The VExUS Nexus. Abhilash Koratala, MD. POCUS J. 2022 Feb 1;7(Kidney):17-20. doi: 10.24908/pocus.v7iKidney.15341. eCollection 2022. [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
- “Abstract: In patients with heart failure and cardiorenal syndrome, lingering congestion is associated with worse outcomes. As such, titrating diuretic or ultrafiltration therapy based on objective assessment of volume status plays a crucial role in the management of these patients. Conventional physical examination findings and parameters such as daily weight measurement are not always reliable in this setting. Recently, point of care ultrasonography (POCUS) has emerged as an attractive enhancement to bedside clinical examination in assessing fluid volume status. Specifically, Doppler ultrasound of the major abdominal veins gives additional information about end-organ congestion when used in conjunction with inferior vena cava ultrasound. Moreover, these Doppler waveforms can be monitored in real time to gauge the efficacy of decongestive therapy. Herein, we present a case that illustrates the utility of POCUS in the management of a patient with heart failure exacerbation.Keywords: VExUS, POCUS, venous Doppler, point of care ultrasound, heart failure”
- Links To And Excerpts From “Unifying Fluid Responsiveness and Tolerance With Physiology: A Dynamic Interpretation of the Diamond-Forrester Classification” With A Link To Dr Rola’s Lecture On VExUS
Posted on February 9, 2024 by Tom Wade MD
Today, I rereviewed this post and reposted to today.
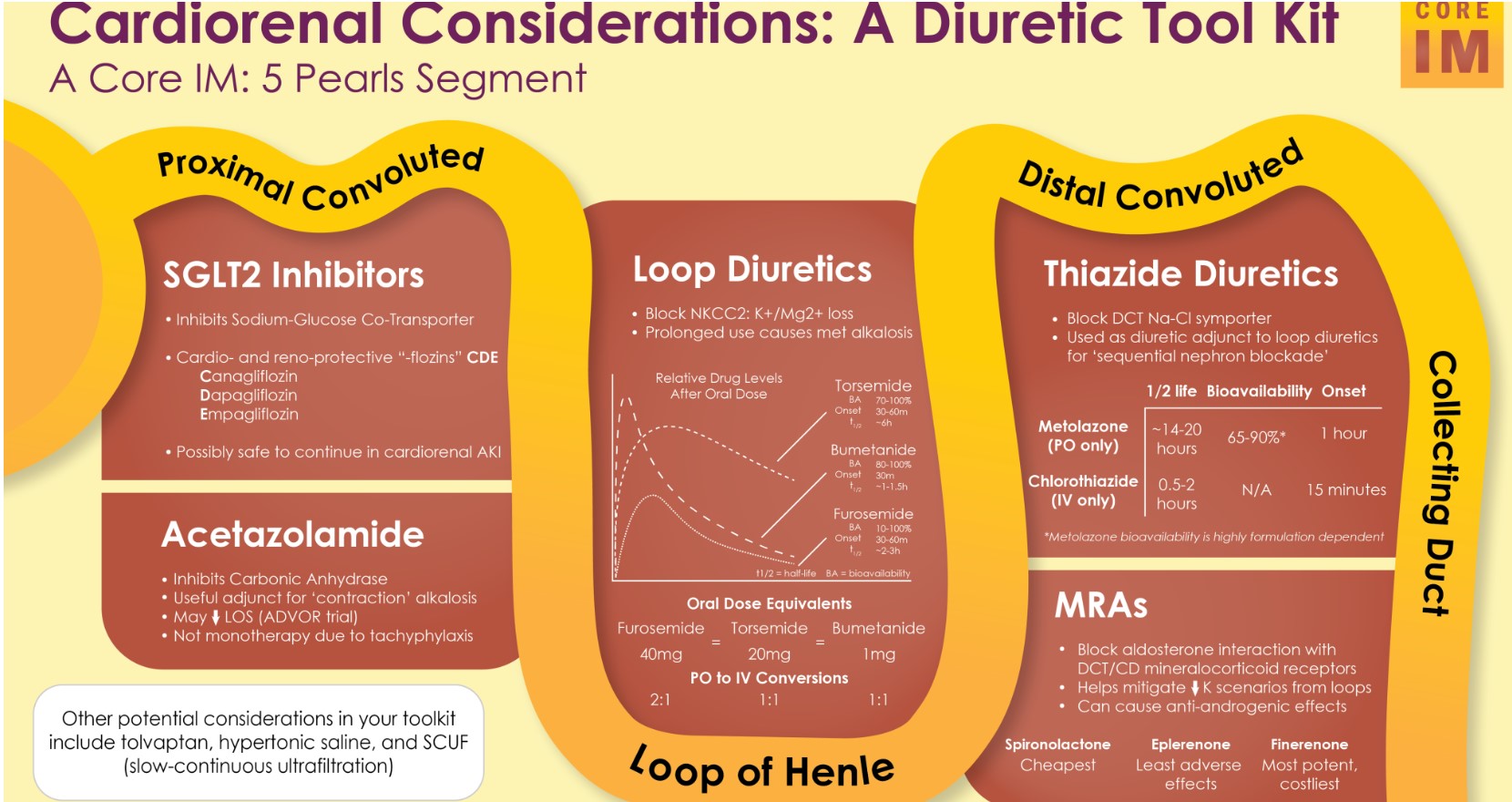
Today, I review, link to, and excerpt from Core IM‘s Cardiorenal Considerations: 5 Pearls Segment. Posted: May 13, 2024
By: Dr. Andrew Ling, Dr. Nayan Arora, Dr. Nicole Bhave and Dr. Shreya P. Trivedi
Graphic: Dr. Rahul Maheshwari
Peer Review: Dr. Larissa Kruger Gomes, Dr. Nisha Bansal
All that follows is from the above resource.
Play Podcast in new window
Time Stamp CME-MOC Show Notes Transcript References
Time Stamps
- 02:05 Pearl 1: Make sure the renal dysfunction is not from something else
- 08:47 Pearl 2: Practical tidbits on loop diuretics
- 18:08 Pearl 3: Assessing diuretic response
- 27:35 Pearl 4: Approaching diuretic resistance
- 35:08 Pearl 5: Don’t be afraid of medical therapy because of CKD
Sponsor:
Freed is an AI scribe that listens, prepares your notes, and writes your note in < 30 seconds. Freed learns your style over time and is HIPAA compliant!
Join over 6K clinicians who love Freed and saves them hours a day! Use the code CORE50 to get 50% off your first month with Freed.
Show Notes
Pearl 1: Make sure the renal dysfunction is actually all cardiorenal!
- How do you define cardiorenal physiology?
- Classic Definition:
- Kidney dysfunction that is related to either a (1) low-flow state and/or (2) renal venous congestion
- BOTH can independently lead to decreased intrarenal blood flow
- BOTH can lead to neurohormonal activation → Increased renin-angiotensin-aldosterone system (RAAS) activity
- These are compensatory mechanisms that initially preserve renal function but can become detrimental when kidney autoregulation can no longer compensate.
- NOTE: Renal venous congestion is thought to be a larger contributor to kidney dysfunction than low-flow states in most cases of cardiorenal syndrome.
- How should you think about the differential for a kidney injury in someone with heart failure?
- Be broad! Multiple processes can happen at the same time
- Urinalysis findings:
- Pure cardiorenal syndrome
- “Bland” with no protein, blood, granular or other cell casts
- May have hyaline casts
- No signs of intrinsic injury!
- NOTE: Screen for proteinuria in heart failure patients!
- Urine protein to creatinine ratio (UPCR) or
- Urine albumin to creatinine ratio (UACR)
- Dilute urine can falsely lower proteinuria on a urine dipstick!
Pearl 2: Practical Tidbits on Loop Diuretics
- What is the “go-to” loop diuretic for someone who is hospitalized for volume overload?
- Intravenous therapy!
- Because gut edema may interfere with oral absorption
- Dosing:
- 2.5 times their equivalent daily oral home dose of diuretic
- Benefits of this dosing:
- Better decongestion at 72 hours
- Faster transition back to oral diuretics
- Compared to lower IV doses
- Continuous vs. Bolus dosing?
- No difference in outcomes!
- NOTE: Patients being treated with bolus may require more escalations in dosing during their hospitalization
- What are the differences between loop diuretics?
- IV formulations:
- Furosemide vs. Bumetanide
- No difference in outcomes demonstrated
- But not largely studied!
- Bumetanide Considerations:
- More potent
- Practitioners may be more comfortable with using higher equivalent doses since bumetanide doses are in the single digits
- Severe myalgias with IV bumetanide as a continuous infusion
- Especially with higher doses
- Unclear if unique to bumetanide or if purely dose-related given furosemide is not typically used at equally high equivalent doses
- PO formulations:
- Furosemide vs. Torsemide vs. Bumetanide
- Conversions (PO): Furosemide 40 mg = torsemide 20 mg = bumetanide 1 mg
- Considerations when choosing PO:
- Bioavailability
- Furosemide
- Torsemide & Bumetanide
- Duration of action
- Torsemide (6-16 hours) > furosemide (6-8 hours) > bumetanide (4-6 hours)
- NOTE: Torsemide can be dosed one daily, whereas
- Bumetanide should be dosed at least twice daily!
- Torsemide is preferred over furosemide since it is more predictable!
- Caveat: The TRANSFORM-HF trial did not show significant difference in outcomes between torsemide and furosemide
- However, there are many criticisms of the trial!
- Significant crossover
- Use of mortality as the primary outcome rather than other metrics
Pearl 3: Assessing Diuretic Response and Renal Function
- How to assess diuretic response?
- Traditional assessment:
- Weights
- Exam findings
- Urine output
- Challenges with relying on these:
- Inaccurate documentation and/or
- Delay in diuretic titration
- In case of inadequate response
- Alternative assessment:
- Spot urine sodium (UNa) level
- Measures diuretic responsiveness
- Quick assessment 1-2 hours after administering loop diuretic
- Interpretation:
- UNa < 50-100 mEq/L → poor response to the current dose of loop diuretic
- GOAL: >70 mEq/L
- For an appropriate response
- Causes of falsely high spot UNa:
- Low volume of urine
- Metabolic alkalosis
- Medications (ex. piperacillin-tazobactam)
- Causes of falsely low spot UNa:
- Cirrhosis
- High volume of urine
- NOTE: Using a spot urine sodium AND 6-hour urine output-guided protocol to escalate loop diuretic dosing was feasible
- What do you do if the response to diuretic is not adequate?
- Double your loop diuretic dose
- Start a drip (if at maximum dose)
- Reassess initial diagnosis or if new insult!
- What happens to creatinine during diuresis?
- Generally safe to see up to 30% rise in from baseline levels with active decongestion
- Known as “rise in serum creatinine” (RSC) or”worsening renal function” (WRF)
- Usually does NOT reflect true renal tubular injury
- Even though this threshold is how acute kidney injury (AKI) is defined
- Rise is a result of:
- Hemodynamic or functional change in glomerular filtration
- Hemoconcentration of creatinine
- Alone, should NOT be a reason to stop diuresing a volume overload patient
- NOTE: Mortality actually worse for patient’s whose serum creatinine “improved” from baseline compared to those who had “worsening” levels.
- The rise has no predictive value for rehospitalization or mortality unless patients also had other signs of congestion at discharge
- Still, always still do the due diligence each day!
- Volume assessment
- Urine output monitoring
- Consider need to evaluate for another cause of rising serum creatinine
- Consider more sophisticated and/or invasive measures of volume status
- Point-of-care ultrasound (POCUS)
- Venous Excess Ultrasound (VExUS)*
- Right heart catheterization
*The above link is not open source. Here are other resources on VEXUS that are open source.
- Evaluation of Venous Congestion Using Beside Ultrasonography by the Nephrology Consultant: The VExUS Nexus. Abhilash Koratala, MD. POCUS J. 2022 Feb 1;7(Kidney):17-20. doi: 10.24908/pocus.v7iKidney.15341. eCollection 2022. [PubMed Abstract] [Full-Text HTML] [Full-Text PDF].
- “AbstractIn patients with heart failure and cardiorenal syndrome, lingering congestion is associated with worse outcomes. As such, titrating diuretic or ultrafiltration therapy based on objective assessment of volume status plays a crucial role in the management of these patients. Conventional physical examination findings and parameters such as daily weight measurement are not always reliable in this setting. Recently, point of care ultrasonography (POCUS) has emerged as an attractive enhancement to bedside clinical examination in assessing fluid volume status. Specifically, Doppler ultrasound of the major abdominal veins gives additional information about end-organ congestion when used in conjunction with inferior vena cava ultrasound. Moreover, these Doppler waveforms can be monitored in real time to gauge the efficacy of decongestive therapy. Herein, we present a case that illustrates the utility of POCUS in the management of a patient with heart failure exacerbation.Keywords: VExUS, POCUS, venous Doppler, point of care ultrasound, heart failure”
- Links To And Excerpts From “Unifying Fluid Responsiveness and Tolerance With Physiology: A Dynamic Interpretation of the Diamond-Forrester Classification” With A Link To Dr Rola’s Lecture On VExUS
Posted on February 9, 2024 by Tom Wade MD
Pearl 4: How to approach diuretic resistance
- What is diuretic resistance?
- Failure to achieve therapeutically desired congestion relief despite using appropriate or escalating doses of diuretics.
- Consider…Is something else going on?
- Diuretics cannot work if they are not reaching the kidney!
- Some factors to consider:
- Shock
- Low-flow state
- Elevated intra-abdominal pressure (ascites)
- How can you augment your diuresis?
- Sequential nephron blockade!
- Thiazide or Thiazide-Like Diuretics:
- PO Metolazone vs. IV Chlorothiazide (or Diuril)
- Metolazone
- Less expensive
- Metolazone has a much longer half-life and duration of action compared to chlorothiazide
- Chlorothiazide
- More expensive (cost is starting to come down)
- NOTE: Many clinicians anecdotally prefer chlorothiazide because of faster onset and IV administration
- Effect:
- No significant difference between two in terms of urine output measured at 24-48 hours.
- Alternative adjusts:
- Hydrochlorothiazide or Chlorthalidone
- Reasonabe options though more in outpatient setting
- Commonly used as antihypertensives
- Acetazolamide
- Achieves uccessful decongestion and shortening length of stay
- Without any differences in safety outcomes!
- In practice: Considered especially in cases of worsening metabolic alkalosis or in COPD
- Caution when using if there is acidosis or serum bicarbonate is low!
- Acute SGLT2i
- Not well-established adjunct
- (though beneficial in general and may be safe to add during heart failure hospitalizations post-AKI)
- Hypertonic saline
- Not well-established adjunct
- What should be monitored during diuresis?
- Electrolytes, particularly hypokalemia
- More common with augmentation
- Hypokalemia is an independent risk factor for development of diuretic resistance
- Add potassium-sparing diuretics early!
- Long-term benefit
- Rapid Volume Depletion
- What about ultrafiltration (UF)?
- CARRESS-HF Trial
- Stepped diuretic algorithm was superior to starting with ultrafiltration
- Preserved renal function
- Lower rates of adverse events
- Criticisms:
- May not be realistic (diuresis was not attempted in UF group)
- Unclear if ideal rate of UF was used
- In practice: UF only after a failing maximal medical therapy
- Due to concerns about future renal function when starting HD and dialysis access complications
Pearl 5: Don’t be afraid of medical therapy because of CKD
- Guideline-directed medical therapy (GDMT) for heart failure
- Also are key medical therapies that slow progression of CKD!
- Benefit of medications seen in patients with:
- Proteinuric CKD (estimated albuminuria > 300 mg/day) with or without diabetes
- GDMT meds are heavily underutilized in patients with concomitant heart failure and CKD
- Despite worse outcomes!
- Percent of patients of GDMT (RAAS inhibition, MRA, beta blocker) by eGFR at discharge
- eGFR 30-45 → only 15% of patients!
- eGFR < 30 → only 5% of patients!
- NOTE: Data from 2014-2019 heart failure registry
- Before SGLT2 inhibitors became an established pillar in heart failure and CKD
- How should you start GDMT in advanced CKD?
- Expect an initial decline in eGFR based on creatinine
- When starting RAAS inhibitors and/or SGLT2 inhibitors
- But don’t panic!
- Decline is usually reflective of:
- Glomerular hemodynamic changes
- Reduction of pathologic hyperfiltration
- Decline is associated with:
- Better outcomes
- Slower annual eGFR decline afterwards
- SGLT2i studies
- General Advice: Tolerate up to a 30% reduction in eGFR after initiation as declines in eGFR larger than 30% are unusual with these agents!
- RAAS Inhibitors
- Tips & Tricks
- RAAS inhibitors should NOT be stopped solely because of declining eGFR
- STOP-ACE Trial – no significant difference in trajectory of GFR decline or number of patients started on RRT when stopping vs. continuing RAS inhibitors at eGFR < 30
- Signal towards worse cardiovascular outcomes, however, when these agents are stopped.
- Hyperkalemia IS more of a reason to pause – but consider trying to treat the potassium first!
- SGLT2 inhibitors are also protective against hyperkalemia – even more reason to have them on board if not already!
- Consider adding potassium binders (see our episode on hyperkalemia in CKD).
- SGLT2 Inhibitors
- Tips & Tricks
- Currently approved for initiation at eGFR > 20
- EMPA-CKD Trial
- Subgroup analyses of DAPA-CKD Trial
- There are ongoing trials where people are being safely continued on these agents even through dialysis!
- SGLT2 inhibitors can likely be continued safely even if eGFR eventually declines to < 20!
- Mineralocorticoid Receptor Antagonists (MRA)
- Tips & Tricks
- All MRAs:
- Improved cardiovascular outcomes and mortality
- At least observational data that they may improve renal outcomes over the long-term
- NOTE:It is still extremely important to closely monitor for dangerous potassium issues and kidney function especially when initiating any MRA!
- Finerenone:
- New agent on the block!
- Slows CKD progression in patients with type 2 diabetes and proteinuric CKD in randomized setting (FIDELIO-DKD)
- Non-steroidal mineralocorticoid receptor antagonist
- More selective for the mineralocorticoid receptor
- Compared to more familiar agents like spironolactone or eplerenone (which are steroidal MRAs)
- Reduced rates of hyperkalemia
- Compared with steroidal MRAs
- Also improves cardiovascular outcomes like the other MRAs (primarily by reduced heart failure hospitalizations)