This post has excerpts from Resource (1) below, Attenuating the Biologic Drive for Weight Regain Following Weight Loss: Must What Goes Down Always Go Back Up? [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Nutrients. 2017 May 6;9(5). pii: E468. doi: 10.3390/nu9050468.
Here is the Abstract and some extracts from Resource (1):
Abstract
Metabolic adaptations occur with weight loss that result in increased hunger with discordant simultaneous reductions in energy requirements—producing the so-called energy gap in which more energy is desired than is required. The increased hunger is associated with elevation of the orexigenic hormone ghrelin and decrements in anorexigenic hormones. The lower total daily energy expenditure with diet-induced weight loss results from (1) a disproportionately greater decrease in circulating leptin and resting metabolic rate (RMR) than would be predicted based on the decline in body mass, (2) decreased thermic effect of food (TEF), and (3) increased energy efficiency at work intensities characteristic of activities of daily living. These metabolic adaptations can readily promote weight regain. While more experimental research is needed to identify effective strategies to narrow the energy gap and attenuate weight regain, some factors contributing to long-term weight loss maintenance have been identified. Less hunger and greater satiation have been associated with higher intakes of protein and dietary fiber, and lower glycemic load diets. High levels of physical activity are characteristic of most successful weight maintainers. A high energy flux state characterized by high daily energy expenditure and matching energy intake may attenuate the declines in RMR and TEF, and may also result in more accurate regulation of energy intake to match daily energy expenditure.
Keywords: weight regain, energy gap, energy intake, energy expenditure, diet composition, exercise
3. Why Is Weight Loss So Difficult to Maintain?
It is estimated that only about 20% of individuals who experience significant weight loss are able to maintain their lost weight [7]. Data from participants in the Biggest Loser Television program demonstrate the difficulty in maintaining lost weight over time. The average weight loss of the 14 participants during the 30-week intervention was 58 kg, but six years later, the contestants had regained an average of 70% of their lost weight (41 kg) [25].
3.1. The Energy Gap Concept
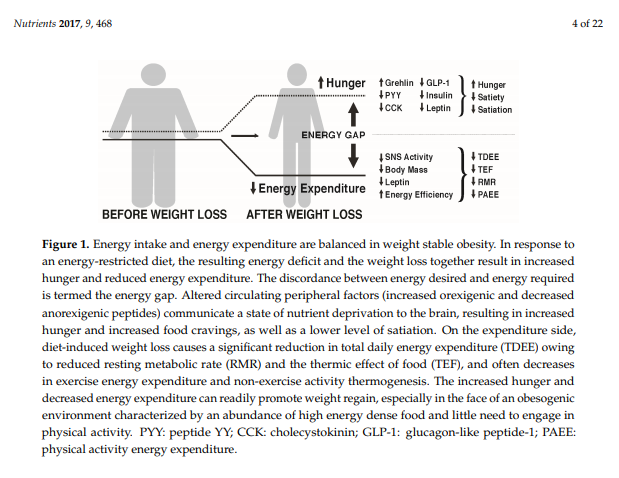
MacLean et al. [27,28] and others [29] have reviewed the metabolic adaptations that accompany weight loss, primarily based on data from animal studies, but with probable relevance to the human condition. As depicted in Figure 1, calorie restriction leading to weight loss causes discordance between appetite and energy requirements, a concept referred to as the “energy gap“, in which the biologic pressure to regain the lost weight occurs as a function of the increased hunger and the reduced energy expenditure that accompany diet-induced weight loss. In response to weight loss, signals of both energy and nutrient deprivation are sent from the periphery to brain networks in the hypothalamus and hindbrain, which by way of second order neurons increase hunger and decrease energy expenditure, resulting in more calories being desired (Ein) than are required (Eout). The occurrence of these responses among individuals living in an obesogenic environment can promote the re-establishment of positive energy balance and regain of body weight and fat toward their pre-diet levels.
3.3. How Does Weight Loss Affect Energy Expenditure?
Total daily energy expenditure (TDEE) is a function of four components: resting metabolic rate (RMR: the energy expenditure required for cellular processes necessary for life as measured when an individual is lying quietly and awake in a post-absorptive state), the thermic effect of food (TEF: the increase in energy expenditure above RMR in response to food ingestion), non-exercise activity thermogenesis (NEAT: energy expenditure above RMR required to support the activities of daily living as well as fidgeting), and exercise energy expenditure (ExEE: energy expended above RMR necessary for performing exercise). NEAT and ExEE together make up physical activity energy expenditure (PAEE). Diet-induced weight loss almost always causes significant decreases in TDEE, which can negatively impact the maintenance of lost weight. As far back as 1984, Leibel and Hirsch reported lower TDEE in post-obese compared to never-obese individuals—on the order of 25% lower than predicted by metabolic body size [60]. In a later study, they determined that obese subjects undergoing 10–20% weight loss experienced a significant decrement in TDEE which could not be entirely explained by the loss of respiring body mass [61]. They reported a mean reduction in TDEE of 8 kcal/kg fat-free mass per day in those obese subjects who lost at least 10% body weight. These decreases in TDEE reflect adaptive thermogenesis (AT)—the change in energy expenditure independent of changes in FFM and the composition of FFM. AT may persist long term [25,61].
The TEF is reduced with dieting because there is a reduction in the total caloric load that requires obligatory digestion and absorption. A portion of the normal TEF results from increased sympathetic nervous system activity that accompanies food ingestion [62], which also decreases with weight loss and lower quantities of food intake.
Numerous studies have shown that weight loss-induced declines in RMR contribute to the reduced TDEE. The reduction in RMR is partially the result of the loss of respiring body mass, but many studies [25,63,64,65], but not all [66], report the magnitude of the decline to be greater than can be explained by the reduction in respiring mass. In the case of diet-induced negative energy balance, a high AT characterized by a reduction in energy expenditure disproportionate to the reduction in FFM (increased resting energy efficiency) attenuates the weight loss drive. The AT associated decrement in RMR could be due to changes in FFM composition, decreases in sympathetic nervous system activity, and lower circulating tri-iodothyronine, leptin, and insulin. Note, however, the magnitude of AT and its contributors appear to vary significantly between individuals and also according to the phase of weight loss. Muller et al. [10] point to the importance of characterizing differences in adaptive thermogenesis during on-going (active) weight loss versus fixed weight loss (maintenance). They suggest that the reduced insulin concentration and changes in the composition of FFM (reduced glycogen and intracellular water) are the primary drivers of AT in the RMR during the first several days of active weight loss (phase 1), but in the second phase as the velocity of ongoing weight loss slows there is little to no AT and the continuing decline in RMR is largely a function of decreased FFM.
There is substantial evidence of AT in regard to RMR with weight loss. For example, Leibel et al. found that a 10–20% weight loss in obese patients caused a decrease in RMR of 3–4 Kcal/kg fat-free mass per day [67]. In the study of Biggest Losers participants, despite the mean weight regain of 41 kg during the six years following the competition, mean RMR of the participants remained 700 kcal/day lower compared to baseline and no different compared to the end of the intervention at 30 weeks [25]. Notwithstanding the sizable inter-individual variation in the magnitude of RMR responses and use of different metabolic carts to measure RMR at six years compared to baseline and 30 weeks, these data provide evidence of significant long-term metabolic adaptation (increased metabolic efficiency) that accompanies weight loss. Even among athletes undergoing significant exercise training, weight loss may result in a decrement in RMR that is disproportionate to the loss of body mass [63,68]. Note that during weight loss maintenance (fixed weight loss) AT is present, with TDEE lower than predicted based on metabolic body size, with this decrement due primarily to the non-resting component as explained below.
The effect of diet-induced weight loss on ExEE and NEAT is quite variable. From a thermodynamic perspective, without changes in movement efficiency and economy, the loss of body mass will result in fewer calories expended to perform the same weight bearing movements. When considering the energy expenditure incurred from activities of daily living at the lower body weight, this could conceivably contribute substantially to the reduction in total daily energy expenditure. Indeed, following a 10% weight loss, individuals were found to exhibit greater skeletal muscle work efficiency at lower intensities, which Rosenbaum et al. estimated could account for one-third of the reduction in PAEE [69]. Others have also found weight loss to result in increased energy efficiency at low exercise workloads [70,71], possibly resulting from the decrease in circulating leptin [70]. There is substantial inter-individual variability regarding changes in energy expenditure and efficiency (i.e., adaptive thermogenesis) with weight loss, both of which are associated with the aforementioned reductions in circulating insulin and leptin. However, two recent studies suggest that changes in the magnitude of circulating leptin and insulin sensitivity alone are not sufficient to explain the magnitude of weight regain [72,73].
To summarize the changes in energy expenditure, diet-induced weight loss can result in significant reductions in RMR, TEF, ExEE, and NEAT. These metabolic changes that result in lower energy expenditure would not obligatorily contribute to weight regain if there were a proportional decrease in food intake. However, as discussed earlier, body composition and hormonal changes occur with weight loss that are associated with increased rather than decreased appetite. This scenario coupled with the environmental pressures of modern society that favor excessive calorie consumption and minimize physical activity, could lead to a sense of futility among both patients and practitioners regarding lifestyle obesity treatment. However, as MacLean et al. [27] suggest, the energy gap “should not be misconstrued into a conciliatory surrender to the inevitability of weight regain”. They instead indicate that the “biological drive to regain lost weight can be countered with environmental, behavioral, and pharmaceutical interventions”. However, they stop short of providing such information in their review. [Emphasis added – I bet the authors were smiling when they wrote the last three sentences.]
4. Can the Weight Loss-Induced Energy Gap Be Attenuated by Lifestyle Factors to Enhance Weight Maintenance?
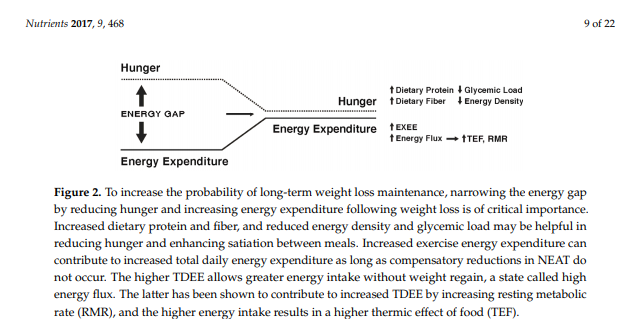
While some individuals are genetically more susceptible to obesity and will likely find it difficult to maintain weight loss [74], there is evidence to suggest that specific strategies and approaches can increase the probability of success. Studies of participants enrolled in the National Weight Control Registry (NWCR, in which enrollees have successfully maintained at least a 13.6 kg weight loss for a minimum of one year, with an average of 33 kg loss maintained for five years [7]) have been useful in identifying predictors of successful weight loss maintenance versus weight regain [75,76,77,78,79,80]. Weight regain has been associated with higher levels of depression, binge eating, dietary disinhibition, increases in hunger, and higher percentage of energy ingested from fat. Predictors of successful weight loss maintenance in the NWCR cohort include frequent weight monitoring, high levels of physical activity, reduced time spent in sedentary activities including television viewing, high levels of dietary restraint, and lower calorie and fat intake. In the most recent longitudinal follow-up of NWCR participants [81], 87% maintained at least a 10% weight loss over a 10-year period. The greatest weight regains occurred in participants who reported large decreases in dietary restraint, physical activity levels, and self-weighing frequency during the first year after weight loss. Note, those enrolled in the NWCR are not representative of all individuals who have attempted weight loss and who have experienced long-term maintenance, but rather are self-selected based on their success. . . . Nevertheless, these data provide evidence that maintenance of lost weight can and does occur in some individuals, even in the face of environmental pressures and well-recognized metabolic adaptations. Figure 2 depicts possible ways to narrow the energy gap by decreasing hunger and increasing energy expenditure.
5. Approaches to Attenuate the Increased Hunger Following Weight Loss
There currently are no clear-cut dietary recommendations for successful weight maintenance following weight loss. In experimental studies examining dietary approaches to curb appetite during weight maintenance, there is substantial inter-individual variability in response to the different diets, suggesting that a one-size-fits-all weight maintenance dietary approach is not likely to be effective for all. Clearly, there is need for more experimental research on dietary patterns and weight maintenance, with special focus on approaches tailored to the unique behavioral and metabolic characteristics of the individual. While much research has focused on understanding the biologic basis for changes in hunger and satiety associated with acute energy perturbations, fewer studies have addressed experimental approaches to reduce hunger over the long-term following weight loss. With this caveat in mind, the following sections provide a brief review of the possible role of diet composition in attenuating the increased hunger that occurs with weight loss. There are other dietary features that might also be useful in minimizing weight regain such as time restricted feeding to better match meal consumption with circadian rhythms [88,89], altering the gut microbiome [89], and consuming water or soups prior to meals [90,91,92], but a discussion of these is beyond the scope of this review.
[These approaches include:]
Diet Composition
Diets of varying macronutrient composition (e.g., high protein; high carbohydrate, low fat; low carbohydrate, ketogenic diets) for weight loss have been the focus of significant research in the past two decades. However, the possible effects of dietary macronutrient composition on weight maintenance following weight loss have received far less research attention, especially in regard to long-term (years) results. Theoretically, a dietary pattern that enhances weight loss maintenance by attenuating the energy gap would result in less hunger and greater satiation relative to energy intake and less reduction in energy expenditure relative to energy intake. Given the greater satiating value of protein compared to other macronutrients [93,94,95] along with its higher thermic effect upon consumption [62], higher intakes of protein may be especially helpful for weight maintenance. Several studies provide experimental evidence in support of the theoretical effects. Westerterp-Plantenga et al. [96] reported that following 5–10% weight loss in overweight and obese men and women, the addition of 48 g/day of protein to the usual dietary intake (total of 18% of energy intake as protein) compared to no additional protein added to usual intake (15% of energy intake as protein) during a three-month weight maintenance period resulted in only half the body weight regain, with the weight regain consisting of fat-free mass. The ‘additional protein’ group also experienced increased satiety and decreased energy efficiency, both of which are important in attenuating the energy gap.
Another aspect of dietary macronutrient composition that may have relevance to attenuation of the weight loss-induced energy gap is the glycemic load, a function of the glycemic index (mathematically derived numerical expression of the ability of the carbohydrates in food to raise blood glucose concentrations following ingestion) multiplied by the total amount of carbohydrate in the diet. Observational studies described in a prior section have found weight loss maintenance to often be associated with consumption of low energy dense foods including vegetables, whole fruits, and legumes, and low carbohydrate foods such as lean meats, which together would constitute a low-to-moderate glycemic load eating pattern. Experimental studies have shown that meals with lower energy density result in lower energy intake [102].
A number of epidemiological studies have found high fiber intakes to be associated with lower food intake and improved weight control [107,108,109]. High fiber diets are thought to evoke a time-energy displacement, which refers to the increased time it takes to chew and consume indigestible carbohydrates (fiber). Slowed eating may allow more time for gastrointestinal peptides to be released during the meal, resulting in earlier meal termination. Related to this concept, meals high in fiber exhibit lower energy density which may decrease energy intake over the course of the meal.
6. Approaches to Attenuate the Decline in Energy Expenditure Following Weight Loss
While exercise is the most obvious volitional approach to increasing energy expenditure, the different constituents of TDEE each represent a potential target for interventions.
Resources:
(1) Attenuating the Biologic Drive for Weight Regain Following Weight Loss: Must What Goes Down Always Go Back Up? [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Nutrients. 2017 May 6;9(5). pii: E468. doi: 10.3390/nu9050468.
(2) Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Nutr Diabetes. 2017 Jun 19;7(6):e282. doi: 10.1038/nutd.2017.31
(3) Successful and unsuccessful weight-loss maintainers: strategies to counteract metabolic compensation following weight loss [PubMed Abstract] [Full Text HTML] [Full Text PDF]. J Nutr Sci. 2018 Jun 28;7:e20. doi: 10.1017/jns.2018.11. eCollection 2018.
(4) Increasing energy flux to decrease the biological drive toward weight regain after weight loss – A proof-of-concept pilot study [PubMed Abstract]. Clin Nutr ESPEN. 2016