This post is the fifth of a series I’ve made for my study notes on Cardiogenic Shock. This post contains links to and excerpts from the Management Of CS section of Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Circulation. 2017 Oct 17;136(16):e232-e268.
The above article has been cited by 38 PubMed Central articles.
Here are excerpts [note to myself, I’ve linked the title headings below directly to the title headings of the article-so I can easily read the full section]:
Reperfusion and Revascularization in CS
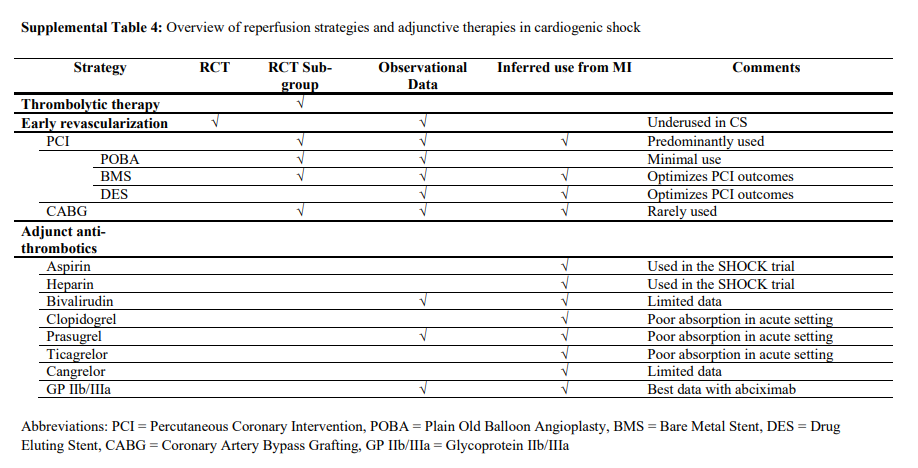
Coronary reperfusion is the mainstay evidence-based therapeutic intervention for patients with acute MI presenting with CS.5,153,154 In this section, reperfusion and revascularization techniques and other adjunctive therapies used in the management of CS are reviewed (Supplemental Table 4).
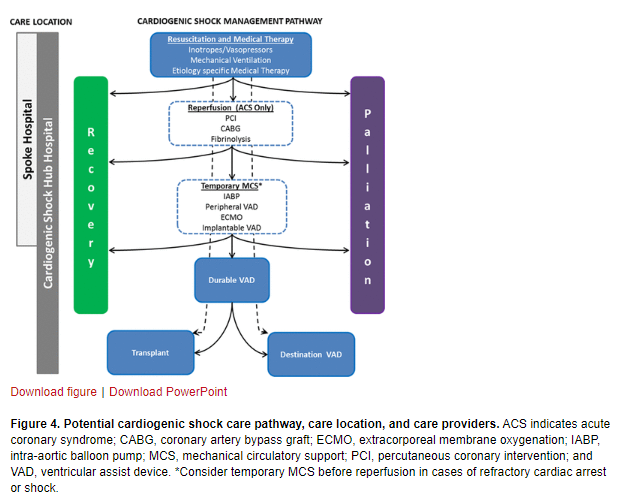
A proposed integrated CS care pathway is outlined in Figure 4.
Fibrinolytic Therapy
The writing group recognizes both the lack of evidence to support fibrinolytic therapy and that timely access to an early invasive approach will not be available to all patients with CS.
Suggestions/Considerations for Clinical Practice
We suggest that when an early invasive approach cannot be completed in a timely fashion, fibrinolysis can be
considered in CS associated with STEMI. The decision to
administer fibrinolysis should be individualized on the
basis of perceived reperfusion benefit, bleeding risks,
and the anticipated time delay to angiography.Early Invasive Strategy in CS
Suggestions for Clinical Practice
We support guidelines that recommend an early invasive strategy with appropriate revascularization for all suitable patients with suspected ACS-associated CS, including patients with uncertain neurological status or those who have received prior fibrinolysis, regardless of the time delay from MI onset.
Suggestions for Clinical Practice
In summary, evidence continues to support the early
revascularization of patients with CS after ACS, with
either PCI or CABG used as indicated. Until the results
of CULPRIT-SHOCK are available, revascularization of
both the culprit and hemodynamically significant nonculprit stenoses is reasonable. We support the preferential use of radial arterial access for angiography and PCI when feasible.Suggestions for Clinical Practice
We suggest that in patients with MI-associated CS who
have multivessel or left main disease, PCI or CABG revascularization decisions should be made collaboratively between cardiologists and surgeons by incorporation of the patient’s medical information, coronary anatomy, procedural risks, potential treatment-related delays, and expressed preferences.Medical Management of the Patient With CS
Critical Care Unit Monitoring and Hemodynamic Goals
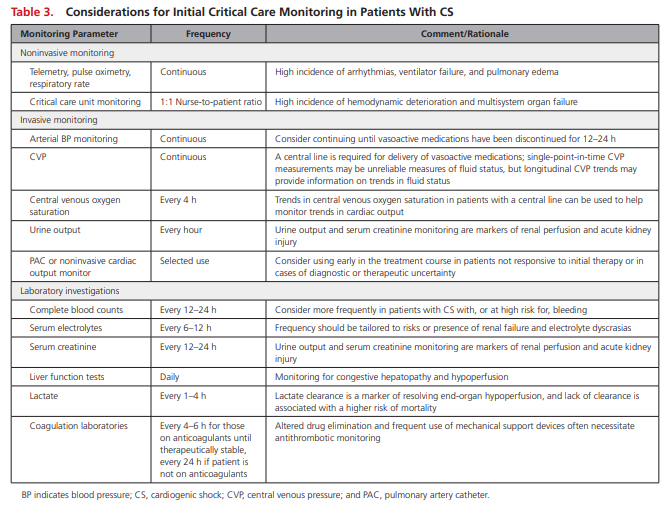
Relatively few data are available to guide appropriate
monitoring decisions for patients with CS. An overview of suggested tools is provided in Table 3 .
The inherent hemodynamic instability and high prevalence of vasopressor use in CS merit invasive arterial blood pressure monitoring to guide drug titration. Central venous catheter insertion should also be considered to support the administration of vasoactive medications and to facilitate monitoring of CVP and mixed central venous oxygen saturation,
which may be helpful in determining the adequacy of tissue oxygen delivery. Clinical examination and laboratory testing are also necessary for monitoring end-organ perfusion and function
[The authors state that] although clinical trials have shown no benefit with the routine use of PAC hemodynamic monitoring, observational studies in CS populations have been mixed, and the PAC remains a potentially important diagnostic and management tool for these individuals.199–202
Hemodynamic data provided by a PAC can confirm the presence and severity of CS, involvement of the RV, pulmonary artery
pressures and transpulmonary gradient, and vascular resistance of the pulmonary and systemic arterial beds.In addition, a PAC may provide CS prognostic information such as CI and cardiac power and enables clinicians to monitor responses to therapeutic interventions.39,203
[Unfortunately], treatment targets are considerably less well established.
Unfortunately, no clear SBP or mean arterial pressure (MAP) suggestions can be made because MAP targets are often extrapolated from non-CS populations in whom a value of 65 mm Hg has been considered a reasonable target.205 CS is a hemodynamically heterogeneous disorder, and hemodynamic variables may not necessarily reflect differential patterns of endorgan blood flow or tissue perfusion.
Microcirculatory dysfunction may persist despite improvements in these hemodynamic measurements.206
Suggestions for Clinical Practice
We suggest the use of PACs in cases of diagnostic or CS management uncertainty or in patients with moderate to severe CS who are unresponsive to initial therapy. Hemodynamic monitoring should complement (and not replace) other markers of end-organ perfusion in CS. The optimal MAP likely differs from patient to patient, and the risks of hypoperfusion with lower MAPs must be balanced (and individualized) with the potentially deleterious impact of vasoactive agents on myocardial oxygen demand, ischemia, and arrhythmia associated with higher MAP targets.
We suggest that clinicians assess the adequacy of end-organ and tissue perfusion in response to individualized targets by integrating serial markers of systemic perfusion, including (but not limited to) arterial lactate, mixed or central venous oxygen saturations, urine output, creatinine, liver function tests, mental status, temperature, and other invasive hemodynamic variables.
Nonvasoactive Pharmacological Management
Considerations for Clinical Practice
We support guideline recommendations for the management of patients with STEMI that suggest avoidance of β-blockers in patients with signs of HF or low-output states and the avoidance of RAAS antagonists in patients with hypotension.144 It may be reasonable to initiate β-blockers when the patient is euvolemic and off inotropes and vasopressors for at least 24 hours. RAAS
inhibitor therapy initiation can be considered when the patient has been off vasopressors for 24 hours, provided that the patient’s renal function has returned nearly to baseline levels and the risk of RAAS-associated hyperkalemia or hypotension is low. RAAS inhibitors may be started in patients with pulmonary edema and in conjunction with an inodilator.We suggest that it is reasonable to administer statin in patients with MI-associated CS.
Vasopressors and Inotropes
Vasoactive medications are often used in the management of patients with CS. An overview of the cardiac
and vascular receptors, along with the hemodynamic
effects of commonly used vasoactive medications in CS,
is provided in Table 4.
Despite their frequent use, few clinical outcome data are available to guide the initial selection of vasoactive therapies in patients with CS.
Dopamine [in the SOAP II trial] was associated with a higher rate of arrhythmias in the CS and overall populations and was associated with higher risk of mortality in the CS subgroup. [But the study has been criticized for methodological flaws.]
Suggestions for Clinical Practice
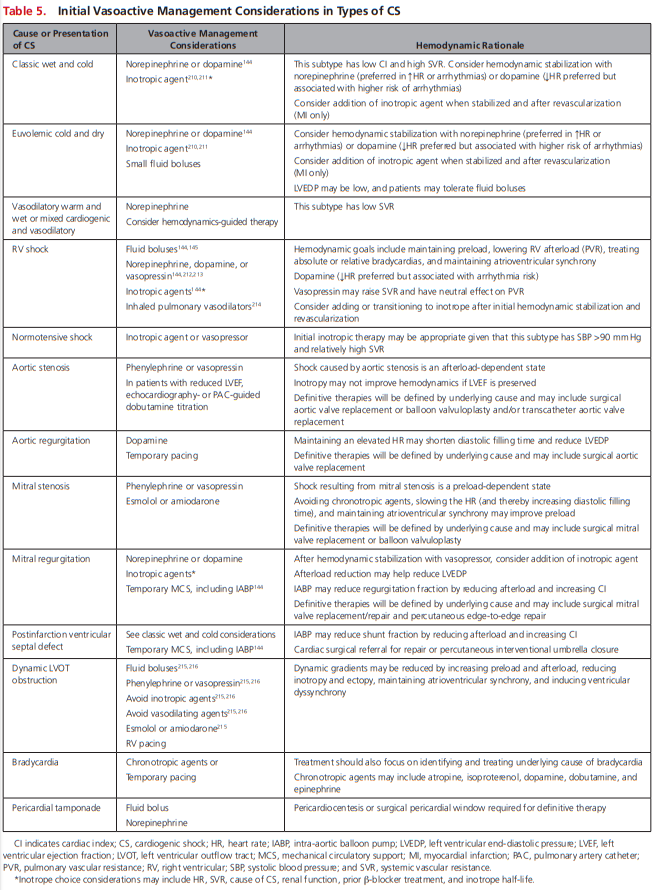
Norepinephrine is associated with fewer arrhythmias and may be the vasopressor of choice in many patients with CS; however, in light of the aforementioned major study limitations, the optimal first-line vasoactive medication in CS remains unclear. Pragmatic initial vasoactive considerations are provided in Table 5.
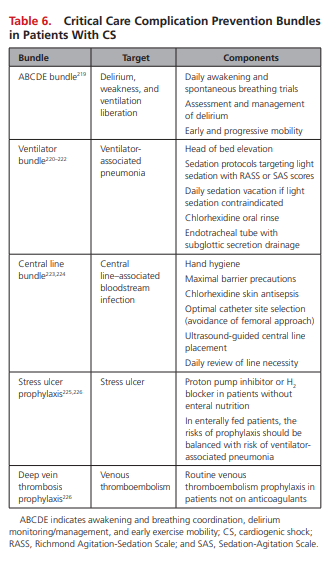
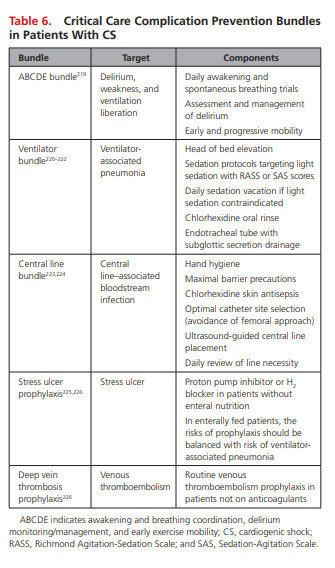
Care Bundles and the Prevention of Critical Care Complications
Although none have been specifically validated among CS cohorts, organizations such as the Institute of Healthcare Improvement recommend the universal use of several of these [Critical Care] bundles in every ICU.218
Suggestions for Clinical Practice
Table 6 highlights key care bundles and prevention practices that should be considered for patients with CS.