Wikipedia’s article on The European Union League Against Rheumatism (EULAR) [This link is to the organization’s home page] states:
The European League Against Rheumatism (abbr. EULAR) is a European non-governmental organization which represents the people with arthritis/rheumatism, health professional and scientific societies of rheumatology of all the European nations.
The aims of EULAR are to reduce the burden of rheumatic diseases on the individual and society and to improve the treatment, prevention and rehabilitation of musculoskeletal diseases.
It promotes the translation of research advances into daily care and fights for the recognition of the needs of people with musculoskeletal diseases by the governing bodies in Europe.[1]
This post has links to and excerpts from EULAR revised recommendations for the management of fibromyalgia [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Ann Rheum Dis. 2017 Feb;76(2):318-328. doi: 10.1136/annrheumdis-2016-209724. Epub 2016 Jul 4.
And here are some excerpts:
Introduction:
Fibromyalgia is common with a prevalence of 2%
in the general population.1 2 However, its diagnosis
and management remain a challenge for patients
and healthcare professionals. It often takes >2 years
for a diagnosis to be made with an average of 3.7
consultations with different physicians.3 Referral to
specialists and investigations results in high healthcare
use, for up to 10 years prior to diagnosis, compared
with persons who do not have fibromyalgia.4 Although pain is the dominant symptom in fibromyalgia,
other symptoms such as fatigue, nonrefreshed
sleep, mood disturbance and cognitive
impairment are common, but not universal, have an
important influence on quality of life and emphasise
that it is a heterogeneous and complex condition.5 6Our aim [is] using the systematic reviews conducted and taking into account their quality, to make evidence-based recommendations for the use of individual pharmacological
and non-pharmacological approaches, and how these
could be combined.Evaluation of pharmacological medicines
Amitriptyline
Five reviews included up to 13 trials and a maximum of 919
subjects. Häuser et al12 reported that patients receiving amitriptyline
were more likely to achieve 30% pain reduction (risk
ratio (RR) 1.60, 95% CI 1.15 to 2.24), equivalent to a ‘number
needed to treat’ (NNT) of 3.54, 95% CI 2.74 to 5.01. There
was a moderate effect on sleep (standardised mean difference
(SMD) −0.56, 95% CI −0.78, to −0.34)i and small effect on
fatigue (−0.44; −0.71 to −0.16). There was no difference in discontinuation
rates compared with patients receiving placebo.
Nishishinya et al13 in their high-quality review concluded that
25 mg/day improved pain, sleep and fatigue at 6–8 weeks of
treatment but not at 12 weeks while 50 mg/day did not demonstrate
efficacy. Amitriptyline evaluation: weak for, at low dose
(100% agreement).Anticonvulsants
Nine reviews of pregabalin included up to seven studies and a
maximum of 3344 patients. A recent Cochrane review24
reported patients receiving active treatment were more likely to have 30% pain reduction, RR 1.37, 95% CI 1.22 to 1.53, with
a ‘number needed to benefit’ (NNTB) over placebo of 9, 95%
CI 7 to 13. There was a very small effect on fatigue (−0.17;
−0.25 to −0.09) and small effect on sleep (−0.35; −0.43 to
−0.27) but no effect on disability (−0.01; −0.11 to 0.09).
A single, moderate quality, study of gabapentin in 150 subjects
(eg, in ref. 104) showed a significant effect on 30% pain reduction
(RR 1.65, 95% CI 1.10 to 2.48), a small effect on sleep
(−0.71; −1.08 to −0.24) and a large effect on disability (−0.94;
−1.32 to −0.56). Anticonvulsant evaluation: pregabalin—weak for (94% agreement); gabapentin—research only (100% agreement).Cyclobenzaprine
A single systematic review of five studies involving 312 patients
reported that of those taking cyclobenzaprine 85% experienced
side effects and only 71% completed the studies. They were
more likely to report themselves as ‘improved’ (NNT 4.8, 95%
CI 3.0 to 11.0). Only two studies reported an ‘intentionto-treat’
(ITT) analysis. Sleep, but not pain, showed a significant,
very small, improvement relative to baseline at the longest outcome considered (12 weeks: SMD 0.34) and patients on placebo showed similar improvement (SMD 0.52).25 Cyclobenzaprine evaluation: weak for (75% agreement).Growth hormone
A single systematic review of two studies involving 74 patients
reported an effect size on pain of 1.36 (0.01 to 1.34).16 The
improvement in functional deficit was not statistically significant
(1.24; −0.36 to 2.84). There are concerns on safety (sleep
apnoea, carpal tunnel syndrome). The drug is not approved for
fibromyalgia (FM) or related disorders in Europe. Growth
hormone evaluation: strong against (94% agreement).Monoamine oxidase inhibitors
Four reviews identified up to three studies and 241 patients.
Häuser et al26 reported a moderate effect on pain across the
studies (−0.54; −1.02, to −0.07), but the single studies that
evaluated fatigue and sleep showed no effect. There were no differences
in dropouts or adverse events compared with placebo.
There was no comparison between compounds. Life-threatening
interactions have been documented. Monoamine oxidase inhibitors.
(MAOIs) evaluation: weak against (81% agreement).NSAIDs
A single review21 identified two small trials with no evidence
of improved outcome compared with placebo. One low-quality
review was not considered. Non-steroidal anti-inflammatory drugs (NSAIDs) evaluation: weak against (100% agreement).Serotonin-noradrenalin reuptake inhibitors
Eight systematic reviews were identified, which presented data
separately for duloxetine. The largest review of 2249 subjects32
reported duloxetine, short term (up to 12 weeks) and long term
(up to 28 weeks), was more effective than placebo at reducing
pain (RR >30% pain, RR 1.38, 95% CI 1.22 to 1.56), although
there was no significant effect at 20–30 mg/day and no difference
between doses of 60 and 120 mg/day. NNTB, based on
60 mg/day up to 12 weeks, was 6, 95% CI 3 to 12. A previous
review reported small effects on sleep (−0.24; −0.37, to −0.12)
and disability (−0.33; −0.43, to −0.24) but no effect on
fatigue.30 Seven systematic reviews were identified of milnacipran, a recent one of which evaluated five trials.30 Patients taking milnacipran were more likely, at the end of treatment, to have 30% pain reduction (RR 1.38, 95% CI 1.25 to 1.51) but there was only a small benefit on fatigue (−0.14; −0.19 to −0.08), disability (−0.16; −0.23 to −0.10) and no effect on
sleep. Duloxetine and milnacipran evaluation: weak for (100% agreement).Selective serotonin reuptake inhibitors
Seven systematic reviews included up to 11 trials and a
maximum of 521 subjects. Given that reviews have not focused
on specific drugs or comparisons, drugs within this class were
considered together. A recent review of medium quality included
seven trials and reported a moderate effect on pain (−0.40; −0.73, to −0.07), sleep (−0.31; −0.60 to −0.02) and no effect on fatigue (−0.17; −0.46 to 0.11).36 Selective serotonin reuptake inhibitor (SSRI) evaluation: weak against (94% agreement).Sodium oxybate – 4-Hydroxybutanoic acid
The European Medicines Agency and the US Food and Drug
Administration refused the approval for FM because of safety
concerns.16 The drug is only approved for narcolepsy. Sodium
oxybate evaluation: strong against (94% agreement).Tramadol, a weak opioid with mild serotonin-noradrenalin
reuptake inhibitor (SNRI) activity was considered by two
reviews. Roskell et al22 identified a single study of tramadol
with paracetamol. Those in the active arm were more
likely to have 30% improvement in pain (RR 1.77, 95% CI
1.26 to 2.48). Tramadol evaluation: weak for (100%
agreement).[Other Medications]
The literature search did not identify any reviews on corticosteroids, strong opioids, cannabinoids and antipsychotics. The committee made a ‘strong against’ evaluation (100% agreement) regarding the use of strong opioids and corticosteroids in patients with fibromyalgia on the basis of lack of evidence of efficacy and high risk of side effects/addiction reported in individual trials.
Evaluation of non-pharmacological therapies; complementary and alternative medicines and therapies
Acupuncture
There is little understanding of the active component
of acupuncture, and the evidence supporting the use of
real versus sham acupuncture was less consistent. Acupuncture evaluation: weak for (93% agreement).Biofeedback
Biofeedback evaluation: weak against (100% agreement).
Capsaicin
Capsaicin evaluation: weak against (86% agreement).
Chiropractic
Chiropractic evaluation: strong against (93% agreement).
Cognitive Behavioral Therapy
Behavioural therapy evaluation: weak for
(100% agreement).Exercise
There is some consistency with regard to aerobic and
strengthening exercises, although insufficient evidence to suggest superiority of one over the other; land and aquatic exercise appear equally effective.56 Exercise therapy evaluation: strong for (100% agreement).Massage
Massage evaluation: weak against (86% agreement).
Meditative Movement
Six reviews, including up to eight trials and 559 participants,
focused on qigong, yoga, tai chi or a combination of these therapies. However, there was insufficient evidence to make individual recommendations. Meditative movement evaluation: weak for (71% agreement).Mindfulness/mind–body therapy
Mindfulness/mind–body therapy evaluation: weak for (73% agreement).
Multicomponent Therapy
Two reviews including up to 27 trials and 2407 participants examined the additional benefit of combining therapies compared with individual therapy. Häuser et al60 conducted a review of management involving both educational or psychological therapies and exercise. In a meta-analysis of nine trials and 1119 patients, multicomponent therapy was effective in reducing pain (−0.37; −0.62 to −0.13), and fatigue, immediately post treatment, compared with waiting list, relaxation, treatment as usual and education. However, effects were short-lived. Multicomponent therapy evaluation:
weak for (93% agreement).Other complementary and alternative therapies
Other complementary and alternative therapies
(guided imagery, homeopathy): strong against (93% agreement).EULAR revised recommendations
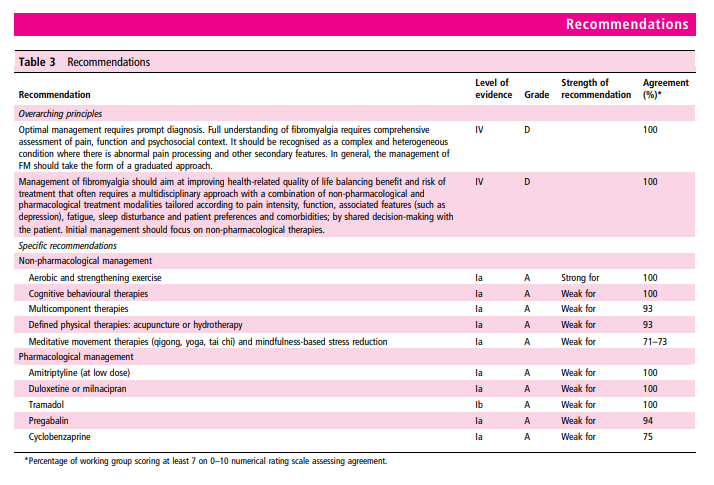
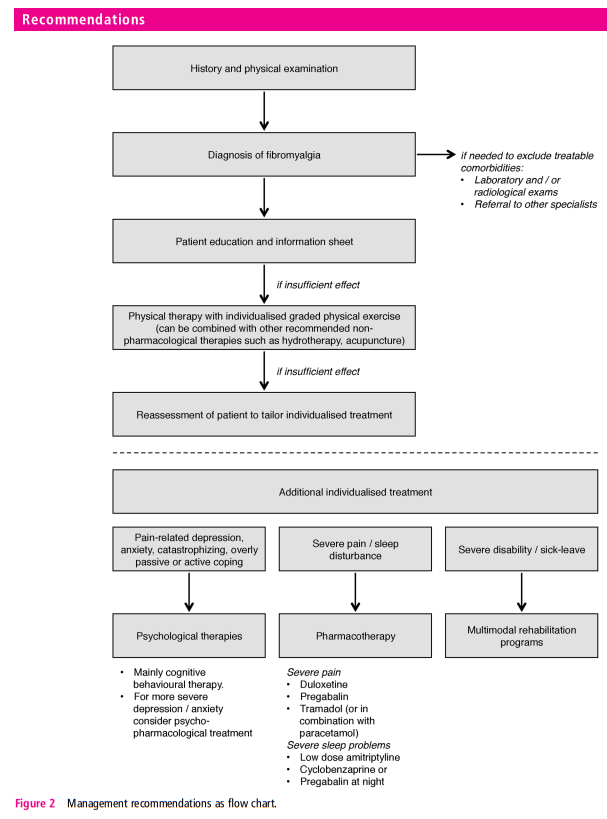
In terms of overall principles, we recommend, based on unanimous expert opinion, that optimal management requires prompt diagnosis and providing the patient with information (including written material) about the condition. There should be a comprehensive assessment of pain, function and the psychosocial context. Management should take the form of a graduated approach with the aim of improving health-related quality of life. It should focus first on non-pharmacological modalities. This is based on availability, cost, safety issues and patient preference. We have used the evaluation of individual therapies (above) to make 10 specific recommendations, all based on evidence from systematic reviews and all but one from meta-analysis. The recommendations are given in table 3, and a flow chart of how these therapies may be used in management is shown in figure 2
In case of lack of effect of the above therapeutic approaches,
we recommend individualised treatment according to patient
need. Psychological therapies (‘weak for’) should be considered
for those with mood disorder or unhelpful coping strategies:
CBT was effective at producing modest, long-term reductions in
pain, disability and improving mood. Pharmacological therapies
(all ‘weak for’) should be considered for those with severe pain
(duloxetine, pregabalin, tramadol) or sleep disturbance (amitriptyline,
cyclobenzaprine, pregabalin). Multimodal rehabilitation
(‘weak for’) programmes should be considered for those with
severe disability—in comparison to individual therapies, those
that were multimodal improved a range of short-term outcomes.
We did not recommend several pharmacological therapies
including NSAIDs, MAOIs and SSRIs because of lack of efficacy
and specifically gave a ‘strong against’ evaluation to growth
hormone, sodium oxybate, strong opioids and corticosteroids
based on lack of efficacy and high risk of side effects.