This post reviews the outstanding RSSDI Therapeutic Wheel. This tool allows us to individualize our therapy for our type 2 diabetics.
Here are the links to the Indian diabetes guidelines, RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017 [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Int J Diabetes Dev Ctries. 2018 Mar;38(Suppl 1):1-115. doi: 10.1007/s13410-018-0604-7. Epub 2018 Feb 28.
The above article has been cited by six articles in PubMed Central.
Here are excerpts from the article:
The RSSDI therapeutic wheel and the excerpts that follow it in this post help us individualize type 2 Diabetes therapy for our patients.
Individualizing therapy
The article is from pp. S50 – SS56 of the PDF. The references for Individualizing Therapy are on pp S54 – S56.
RSSDI 2017 recommendations
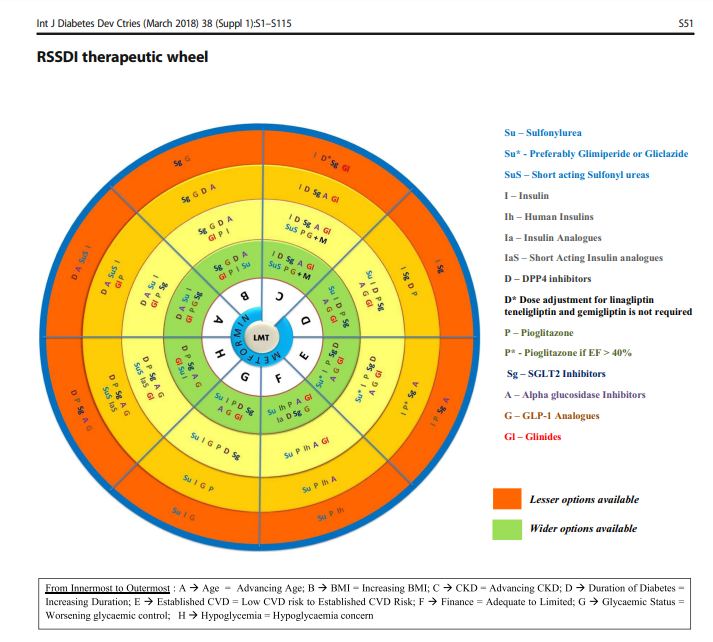
ABCD (EFGH) approach for diabetes management
Choice of any antidiabetic agent should take into account the
patient’s general health status and associated medical disorders. This patient-centric approach may be referred to as the
ABCD (EFGH) approach for diabetes management. As
shown in the figure, for any T2DM patients, first line of therapy should be metformin unless it is not tolerated by the patient or contraindicated.
- For patients who have been diagnosed with diabetes, consider a combination of metformin and one of the treatment options based on patients Age, BMI, CKD, Duration of diabetes, Established CVD, Financial condition, Glycemic status, and Hypoglycemia concern.
- Drug choice should be based on patient preferences as well as presence of various comorbidities and complications and drug characteristics, with the goal of reducing blood glucose levels while minimizing side effects, especially hypoglycemia and weight gain.
- A comparative effectiveness meta-analysis suggests that overall each new class of non-insulin agents added to initial therapy lowers A1C around 0.9–1.1% [1]. Moreover, current National Insulin Summit (NIS) consensus from India reports that all oral antidiabetic agents can reduce A1C to a range of 0.5–2.0% and injectables (GLP-1 RA and insulin) reduce A1C to a range of 0.5–3.5% when used as monotherapy [2].
Note: Hydroxychloroquinone has recently been approved
by DCGI for the treatment of T2DM as third-line therapy;
SGLT2 inhibitors are recommended in patients with high
CV risk.Age
- GFR-adjusted doses of gliptins may be a suitable addition to metformin for elderly patients to avoid hypoglycemia and weight gain [3]. Recent double blind RCTs have reported that gliptins are efficacious and safe with no tolerability issue when used as add-on therapy in elderly patients with T2DM [4–7].
- Agents belonging to AGIs could also be an important
choice in elderly patients. These agents have moderate
efficacy and minimal side effects like hypoglycemia, but
the major limiting factor for their use is the gastrointestinal side effects, such as flatulence and diarrhea [8]. A double blind RCT revealed that compared to diet alone, addition of acarbose improved the glycemic profile and insulin sensitivity in elderly patients with T2DM [9].- Glitazones are a safer alternative in patients with preserved cardiac function. However, postmenopausal females must be spared for their use because of high
predisposition to osteoporosis. Furthermore, the use
of glitazones is restricted in elderly T2DM patients
owing to the complications like weight gain, fluid retention, peripheral edema, aggravation of congestive heart failure, and especially increased risk of bladder cancer associated with their use [10].- Newer sulfonylureas like gliclazide MR and glimepiride (due to low risk of hypoglycemia) and glinides (due to shorter half-life) can be safely used in elderly patients with T2DM [11]. A recent RCT (GENERATION) did not find any significant difference between saxagliptin and glimepiride in elderly T2DM patients [12].
- Evidence regarding the use of GLP-1 RA and SGLT2
inhibitors in elder T2DM patients is limited. However,
available data suggests that agents of both the classes
provide good glycemic control in patients with T2DM.
However, certain drawbacks like cost, injection, and
limited availability with GLP-1RAs and increased risk
of genital and urinary tract infections, hypovolemia,
postural hypotension, and weight loss with SGLT2
inhibitors may limit their usage in some older T2DM
patients [10].- Early initiation of insulin in older patients was found to be beneficial without increasing risk of hypoglycemia or greater total direct healthcare costs [13]. Evidence suggests that basal insulin analogues like glargine and detemir were effective and safe without any risk of hypoglycemia and weight gain [14, 15]. Moreover, a pooled analysis from five RCTs revealed that addition of insulin glargine compared to NPH insulin to oral antidiabetic drugs in older adults was effective with low risk of hypoglycemia [16].
- In addition, individualization of therapy is desirable based on risk of hypoglycemia, comorbidities, functionality, cost, and personal preference.
BMI
- While prescribing pharmacological treatments for overweight or obese patients with T2DM, providers should first consider antidiabetes medications which cause either weight neutrality or weight loss. Metformin, AGIs, GLP-1 RAs, and SGLT-2 inhibitors are associated with weight loss characteristics, and DPP-4 inhibitors appear to be weight neutral [17, 18]. A systematic review and metaanalysis of 62 randomized trials revealed that, when compared to other antidiabetic agents, SGLT2 inhibitors and GLP-1 RAs were associated with clinically significant body weight loss (range, 1.15–2.26 kg) as add-on to metformin [19].
- GLP-1 RA seems to be the best add-on therapy for those having high BMI. This group of medications has the highest weight-reducing property in addition to excellent efficacy. A recent systematic review and mixed treatment comparison meta-analysis report that GLP-1 RAs are associated with weight loss (− 1.62 to − 1.01 kg) in overweight or obese patients with T2DM with no difference in weight loss between different types of GLP-1 RAs [20].
- SGLT-2 inhibitors also have a weight reduction property. Evidence suggests that SGLT-2 inhibitors were associated with weight loss in patients with T2DM [21, 22]. Medicines in this group have an additional advantage of excellent tolerance and can be given orally. However, their glycemic efficacy seems to be less than that of GLP-1 RA and also experience with this group of agents is less than that with GLP-1 R[23].
- Gliptins are weight neutral and so can be used as third line of agents [24, 25].
- Use of newer sulfonylureas compared to older sulfonylureas and other OADs does not result in significant weight gain in patients with T2DM [26–28]. Last option for such kind of patients should be insulin or glitazones since they are having weight gain properties.
Complications (CKD)
- In the same manner, if we focus on complications (renal impairment), preference of therapy would be gliptins as add-on therapy with metformin [29]. Few of the gliptins need dose adjustment as per eGFR while vildagliptin needs dose adjustment in hepatic insufficiency. Linagliptin and teneligliptin do not require any dose adjustment in renal disease [30–33].
- Repaglinide is another agent which may be used across all stages of renal insufficiency. Similarly, glitazones may be used in CKD; however, one has to be careful about fluid retention [34, 35].
- Short-acting sulfonylureas like glipizide can be preferred in patients with moderate/severe renal impairment. Furthermore, in mild/moderate renal impairment, gliclazide and glimepiride may also be used, preferably at lower doses [11].
- GLP-1 RAs, owing to their GI adverse effect, limit their
use in renal insufficiency patients [34].- AGIs may be used in patients with mild to moderate renal disease [35].
- Insulin may be used in any stages of renal insufficiency
and is the best agent for this purpose. Short-acting insulin analogues are preferred over conventional insulins [36] and insulin doses should be reduced with falling eGFR and A1C targets can be increased slightly [37, 38].Duration of diabetes
- Patients with long-standing T2DM are very challenging to treat because these patients often lack sufficient β-cell function to respond to some oral glucose-lowering agents, may have profound comorbidities, and may have renal impairment [39]. As results of recent trials have suggested to utilize an aggressive approach in cases where duration of diabetes <5 years, SU or glinide, as an add-on therapy to metformin, will be the best choices, as they are very potent agents [40]. Addition of glitazones may also be useful at this stage [41].
- Apart from this, ADA 2013 stated that lean patients with long duration of disease may benefit from gliptins or sulfonylureas with early use of insulin [42].
- Basal insulin analogues are often used in patients with
long-standing diabetes to address insulinopenic states
[39].- Incretin-based therapies, particularly GLP-1 receptor agonists, provide postprandial control with lower risks of hypoglycemia than prandial insulin [39]. GLP-1 RA may score over gliptins for this indication as they are more effective than gliptins. Therefore, gliptins may be considered as second add-on option.
- SGLT-2 inhibitors may also be useful as second add-on
agent due to their insulin-independent action which is
pathophysiologically different [43].- AGIs are last choices due to their moderate efficacy.
Established CVD
- Intensive glycemic control with antidiabetic drugs reduces cardiovascular risk and complications in patients with T2DM [44].
- In patients with established CVD, DPP4 inhibitors, GLP-1 analogues, and SGLT-2 inhibitors may be preferred [45].
- Pioglitazone should not be used in heart failure [45] or patients with low ejection fraction [46]. Moreover, pioglitazone has been shown in different studies to reduce CVD risk [47, 48].
- Glimepiride and gliclazide MR can be preferred over conventional sulfonylureas in patients at increased risk of CVD or with CVD [11].
- GLP-1 RAs may be suitable alternative for patients wh0 are overweight or obese. AGIs may be preferred in patients with postprandial hyperglycemia.
- Recent data from EMPA-REG and CANVAS studies have shown that SGLT-2 inhibitors reduce CV risk and CV mortality and may be preferred [49, 50].
Financial Concern
- Considering that many Indian patients do not have medical insurance and treatment needs to be continued lifelong, cost of therapy also plays an essential role in T2DM patients from Indian subcontinent.
- SUs should be the first choice with metformin by considering their cost. Then after AGIs or glitazones should be used at next therapy level [51]. In the next level, the therapeutic option should be glinides or insulin.
- High cost will prevent the use of insulin analogues,
gliptins, SGLT-2 inhibitors, and GLP-1 RA in most of
the patients [52].Glycemic status
- Good glycemic control of patients is directly correlated
with efficacy of any antidiabetic agent.- The order of glucose-lowering agents according to their
efficacy of A1C reduction is insulin, sulfonylureas, GLP-1 RAs, pioglitazone, gliptins, SGLT-2 inhibitors, and AGIs [2, 53].- Insulin followed by GLP-1RA, SUs, and glitazones have
highest efficacy in terms of reducing A1C [54].- As a second-line agent, insulin should be preferred, followed by GLP-1 RA, sulfonylureas, gliptins, and others [55].
- Gliptins, SGLT-2 inhibitors, or AGIs should be considered as add-on therapy if these agents are not able to achieve glycemic targets.
- It is always to be understood that good efficacy, in most
cases, comes with a price written on it in the form of
increased incidence of hypoglycemia or prohibitive cost.Hypoglycemia concern
- Hypoglycemia is the biggest hurdle that any medical fraternity is facing during treatment course of diabetes.
- Sulfonylureas have an increased risk of severe hypoglycemia compared with metformin or thiazolidinedione monotherapy. Moreover, sulfonylureas as a second-line agent have a greater risk of severe hypoglycemia than DPP-4
inhibitors and SGLT-2 inhibitors [53].- A traditional meta-analysis reported that only sulfonylureas (relative risk (RR), 4.57) and glinides (RR, 7.50) were associated with increased risk of hypoglycemia, whereas thiazolidinediones (RR, 0.56), AGIs (RR, 0.42), DPP-4 inhibitors (RR, 0.63), and GLP-1 RAs (RR, 0.89) were not associated [56].
- On introducing DPP-4i on a background of secretagogues, the dose of secretagogues needs to be reduced and close monitoring of blood glucose is necessary [57]. Similarly, while introducing SGLT-2i on a background of insulin or secretagogues, the dose of insulin or secretagogues needs to be reduced [58].
- In patients with history of hypoglycemia or for those at
high risk of hypoglycemia, GLP-1 RA or gliptins should
be considered as first choice with metformin [59]. Other
options include SGLT-2 inhibitors, glitazones, and AGIs.- Last option for such patients should be glinides, sulfonylureas, or insulin since there are high chances of hypoglycemia with these agents.
- Patients requiring to avoid hypoglycemia include:
– Those with established CV disease
– Elderly patients
– Those suffering from retinopathy and cannot perform SMBG without help of others
– Those who stay alone, especially in remote areas
– Those who are having poor longevity
– Those who are having documented hypoglycemia
unawareness
– Those who met with severe symptomatic hypoglycemia requiring hospitalization
The references for Individualizing Therapy are on pp S54 – S56.
Additional Resources:
(1) American association of clinical endocrinologists and american college of endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan – 2015. [PubMed Abstract] [Full Text HTML] [Full Text PDF]. Endocr Pract. 2015 Apr;21 Suppl 1:1-87. doi: 10.4158/EP15672.GL