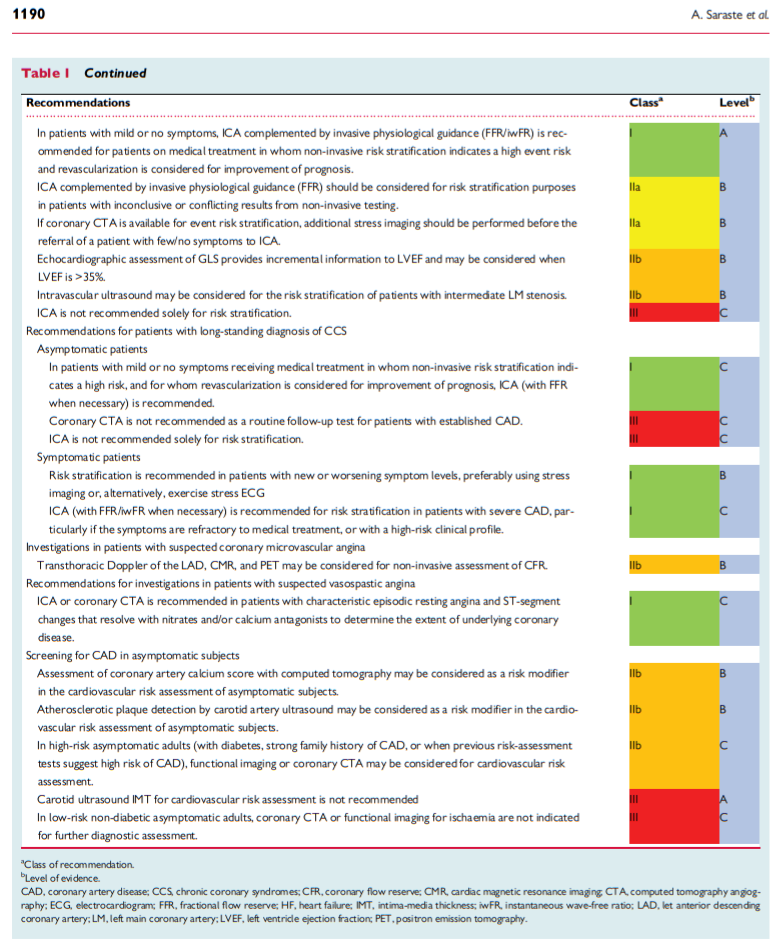
In this post I link to and excerpt from the Imaging in ESC clinical guidelines: chronic coronary syndromes [PubMed Abstract] [Full Text HTML] [Full Text PDF]. European Heart Journal – Cardiovascular Imaging, Volume 20, Issue 11, November 2019.
Article Contents
What follows are excerpts from the above article:
Introduction
Assessment of symptomatic patients with suspected obstructive coronary artery disease (CAD) is a common clinical task and a major component of healthcare cost. Due to variable and often atypical symptoms, objective tests are most often necessary to confirm the diagnosis, exclude alternative diagnoses, and assess the severity of underlying disease. The European Society of Cardiology (ESC) recently published the 2019 ESC guidelines on the diagnosis and management of chronic coronary syndromes (CCS).1 In this article, we will highlight the major new or revised concepts in these guidelines, as they relate to the use of imaging tests for the assessment of patients with suspected or known CCS.
Patient population
The 2019 guidelines focus on the spectrum of CCS rather than solely on stable CAD.1 This change emphasizes the fact that the clinical presentations of CAD can be categorized as either acute coronary syndromes (ACS) or CCS. CAD is a dynamic process of atherosclerotic plaque accumulation and functional alterations of coronary circulation that can be modified by lifestyle, pharmacological therapies, and revascularization which result in disease stabilization or regression patterns.1 In the new guidelines, we identified six clinical scenarios most frequently encountered in practice: (i) patients with suspected CAD and ‘stable’ angina symptoms and/or dyspnoea; (ii) patients with new onset of heart failure or left ventricular (LV) dysfunction and suspected CAD; (iii) asymptomatic and symptomatic patients with stabilized symptoms <1 year after an ACS or patients with recent revascularization; (iv) asymptomatic and symptomatic patients >1 year after initial diagnosis or revascularization; (v) patients with angina and suspected vasospastic or microvascular disease; as well as (vi) asymptomatic subjects in whom CAD is detected at screening.
Diagnostic approach
The diagnostic approach in a patient with suspected obstructive CAD can be divided into a series of subsequent steps.1 Step 1 is to assess the symptoms and signs to identify patients with possible unstable angina or other forms of ACS. If an ACS is suspected, the further course should proceed according to the respective guidelines.2 In patients without unstable angina or other ACS, step 2 is to evaluate the patient’s general condition and quality of life. Comorbidities that could potentially influence therapeutic decisions are to be assessed and other potential causes of the symptoms are considered. Step 3 includes basic testing and assessment of LV function. A resting transthoracic echocardiogram is recommended in all patients for exclusion of alternative causes of angina, identification of regional wall motion abnormalities suggestive of CAD, determination of left ventricular ejection fraction (LVEF) for risk-stratification purposes as well as evaluation of diastolic function (Table 1). Cardiac magnetic resonance (CMR) imaging may be considered in patients with an inconclusive echocardiographic examination (Table 1).
In step 4, the pre-test probability (PTP) and clinical likelihood of
obstructive CAD are estimated and, on this basis, diagnostic testing strategies, either non-invasive or invasive, are offered to selected patients to establish the diagnosis of CAD (step 5). Once the diagnosis of obstructive CAD has been confirmed, the patient’s event risk will be determined (step 6). Risk stratification has major impact on therapeutic decisions, in particular, identification of patients at high event risk who will benefit from revascularization beyond the amelioration of symptoms. Event risk stratification is recommended based
on clinical assessment and the result of the diagnostic test initially employed for making a diagnosis of CAD (Table 1). Risk assessment includes evaluation of LVEF by echocardiography in all patients. Systolic function can be reduced without a decrease in LVEF, and a decreased global longitudinal strain (GLS) by >2 standard deviations from the lower normal reference value has demonstrated incremental value in risk assessment of patients with CCS, especially in those with LVEF >35%.3–5PTP and clinical likelihood of CAD
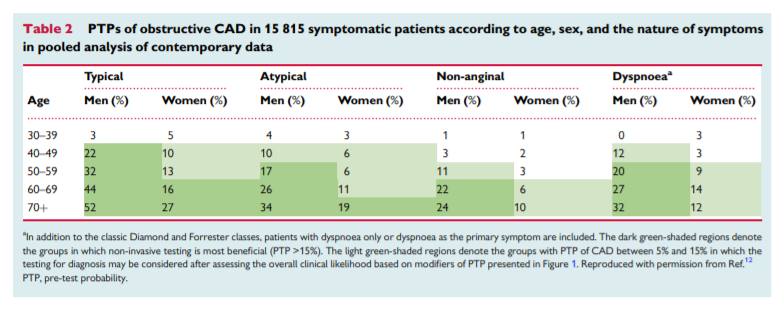
Estimation of the PTP and clinical likelihood of obstructive CAD serves to identify patients who require further investigation or treatment, and to distinguish them from those who do not need further investigation. As in the previous version of the guidelines, determination of the PTP of obstructive CAD by the practical clinical prediction model incorporating age, sex, and nature of symptoms remains the main component of this process in the new guidelines (Table 2).1
Application of the new PTP (Table 2) has important consequencesfor the referral of patients to diagnostic testing.1 If diagnostic testing was deferred in patients with new PTP <15%, this would result in a large increase in the proportion in whom diagnostic testing is not recommended. In data derived from the PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial, 50% of patients previously classified as having an intermediate likelihood of obstructive CAD were reclassified to a PTP <15% according to the new PTP.9 In data derived from the pooled analysis,12 57% of all
patients were classified to a PTP <15%.Studies have shown that outcome in patients with the new PTP up to 15% is good (annual risk of cardiovascular death or myocardial infarction (MI) <1%).9,11 Hence, it is safe to defer routine testing in patients with PTP <15%, thus reducing unnecessary procedures and costs. However, currently there are no randomized controlled trials that include evaluation of outcomes with ‘no-test’ strategy.
Recent studies have demonstrated that when tested, the true
observed prevalence of obstructive CAD has been <5% in patients who had PTP <15% according to the 2013 version of these guidelines.10,11 Therefore, performing diagnostic testing also in patients with a new PTP of 5–15% more closely reflects current clinical practice and may be considered appropriate, in particular, if symptoms are limiting and require clarification.1 Patient preference, local resources and availability of tests, clinical judgement, and appropriate patient information remain important for the decision to proceed with noninvasive diagnostic testing in an individual patient when the PTP is 5–
15%, and the higher likelihood of a false-positive test must be considered. A practical and reasonable approach would be to assume that patients with a PTP <_5% can be assumed to have such a low probability of disease that diagnostic testing should be performed only for compelling reasons (such as additional signs and symptoms not reflected in the guidelines).A new phrase ‘Clinical likelihood of CAD’ that incorporates modifiers of PTP other than age, sex, and nature of symptoms was introduced in the new guidelines.1 Clinical models that incorporate information on risk factors for cardiovascular disease, resting electrocardiogram (ECG) changes, or coronary calcification provide improved identification of patients with obstructive CAD compared with age, sex, and symptoms alone.11,14–20 Therefore, the presence of risk factors for cardiovascular disease (such as family history of cardiovascular disease, dyslipidaemia, diabetes, hypertension, smoking, and other lifestyle factors) that increase the probability of obstructive CAD can be used as modifiers of the PTP estimate.1 If available, Q-wave or ST-segment or T-wave changes on the ECG, LV dysfunction suggestive of ischaemia, and findings on exercise ECG as well as information on coronary calcium by computed tomography can also be used to improve estimations of PTP of obstructive CAD.1 In particular, the absence of coronary calcium (Agatston score = 0) is CAD can be used as modifiers of the PTP estimate.1 If available, Q-wave or ST-segment or T-wave changes on the ECG, LV dysfunction suggestive of ischaemia, and findings on exercise ECG as well as
information on coronary calcium by computed tomography can also be used to improve estimations of PTP of obstructive CAD.1 In particular, the absence of coronary calcium (Agatston score = 0) is associated with a low prevalence of obstructive CAD (<5%) and risk of death or non-fatal MI (<1% annual risk).20,21 However, it is notable that the absence of coronary calcium does not exclude coronary stenosis caused by a non-calcified atherosclerotic lesion,21 and the presence of coronary calcium as such is a weak predictor of obstructive CAD.20 Although the optimal use of these factors in improving PTP assessment has not yet been established, the guidelines indicate that they should be considered in addition to the PTP based on sex, age, and nature of symptoms to determine overall clinical likelihood
of obstructive CAD, as summarized in Figure 1. This is particularly important in refining the likelihood of CAD patients with PTP of 5– 15% based on age, sex, and nature of symptoms alone.Place fig4 when server not busy.
Diagnostic tests
Start here