For an outstanding brief review of this topic please see also
EMCrit 259 – Cardiogenic Shock — The Next Level & Mechanical Circulatory Support with Jenelle Badulak. November 13, 2019 by Scott Weingart, MD FCCM.*
*Scott Weingart, MD FCCM. EMCrit 259 – Cardiogenic Shock — The Next Level & Mechanical Circulatory Support with Jenelle Badulak. EMCrit Blog. Published on November 13, 2019. Accessed on August 1st 2021. Available at [https://emcrit.org/emcrit/cardiogenic-shock-2-mcs/ ].
In this post, I link to and excerpt from Contemporary Management of
Cardiogenic Shock: A Scientific Statement From the American Heart Association [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Circulation. 2017 Oct 17;136(16):e232-e268.
The above article has been cited 179 articles in PubMed.
Be sure and download the supplementary material of the article [PDF] which includes:
- Supplemental Table 1: Etiologies of cardiogenic shock
- Supplemental Table 2: Utility of the echocardiogram in cardiogenic shock
- Supplemental Table 3: Potential cardiogenic shock systems of care implementation barriers and solutions
- Supplemental Table 4: Overview of reperfusion strategies and adjunctive therapies in cardiogenic shock
- Supplemental Table 5: Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles
- Supplemental Table 6: Comparison of commonly percutaneous mechanical circulatory support devices
- Supplemental Table 7: Objective, subjective and patient-centric criteria to guide palliative care readiness and/or discussions and services
- Supplemental Table 8: Characteristics of patients hospitalized with acute coronary syndromes who received palliative versus conservative or reperfusion treatments
- Supplemental Appendix 1: Description of veno-arterial extra-corporeal membrane oxygenation (ECMO)
Jump to
- Abstract
- Definition of CS
- Historical Perspectives
- Pathophysiology
- Hemodynamic Phenotypes
- Pathogenesis
- Laboratory Evaluation, Noninvasive Testing, and Hemodynamic Monitoring
- Contemporary Outcomes, Prognosis, and Resource Use
- Regionalized Systems of Care
- Management of CS
- Palliative Care in CS
- Future Directions
- Conclusions
- Acknowledgment
- Disclosures
- Footnotes
- References
- Supplementary Materials
All that follows is from the above article.
ABSTRACT: Cardiogenic shock is a high-acuity, potentially complex, and hemodynamically diverse state of end-organ hypoperfusion that is frequently associated with multisystem organ failure. Despite improving survival in recent years, patient morbidity and mortality remain high, and there are few evidence-based therapeutic interventions known to clearly
improve patient outcomes. This scientific statement on cardiogenic shock summarizes the epidemiology, pathophysiology, causes, and outcomes of cardiogenic shock; reviews contemporary best medical, surgical, mechanical circulatory support, and palliative care practices; advocates
for the development of regionalized systems of care; and outlines future research priorities.DEFINITION OF CS
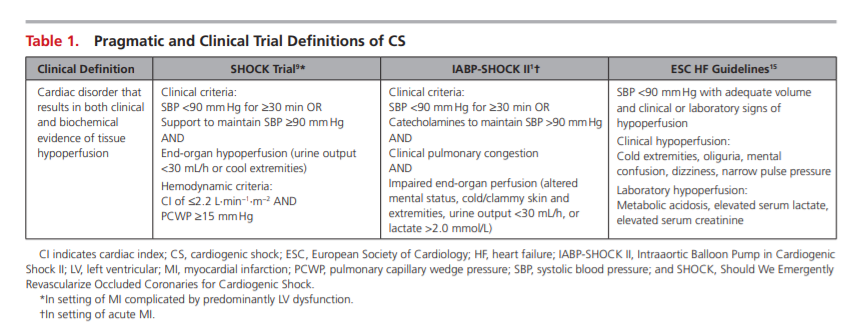
Acute cardiac hemodynamic instability may result from disorders that impair function of the myocardium, valves, conduction system, or pericardium, either in isolation or in combination. CS is pragmatically defined as a state in which ineffective cardiac output caused by a primary cardiac disorder results in both clinical and biochemical manifestations of inadequate tissue perfusion. The clinical presentation is typically characterized by persistent hypotension unresponsive to volume replacement and is accompanied by clinical features of end-organ hypoperfusion requiring intervention with pharmacological or mechanical support. Although not mandated, objective hemodynamic parameters for CS can help
confirm the diagnosis and enable comparison across
cohorts and clinical trials. Definitions in clinical practice
guidelines and operationalized definitions used in the
SHOCK (Should We Emergently Revascularize Occluded
Coronaries for Cardiogenic Shock) and IABP-SHOCK II
(Intraaortic Balloon Pump in Cardiogenic Shock II) trials
are presented in Table 1.1,9,15*
*For the most current definitions of Cardiogenic Shock, please see SCAI clinical expert consensus statement on the classification of cardiogenic shock [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Catheter Cardiovasc Interv. 2019 Jul 1;94(1):29-37.
PATHOPHYSIOLOGY
HEMODYNAMIC PHENOTYPES
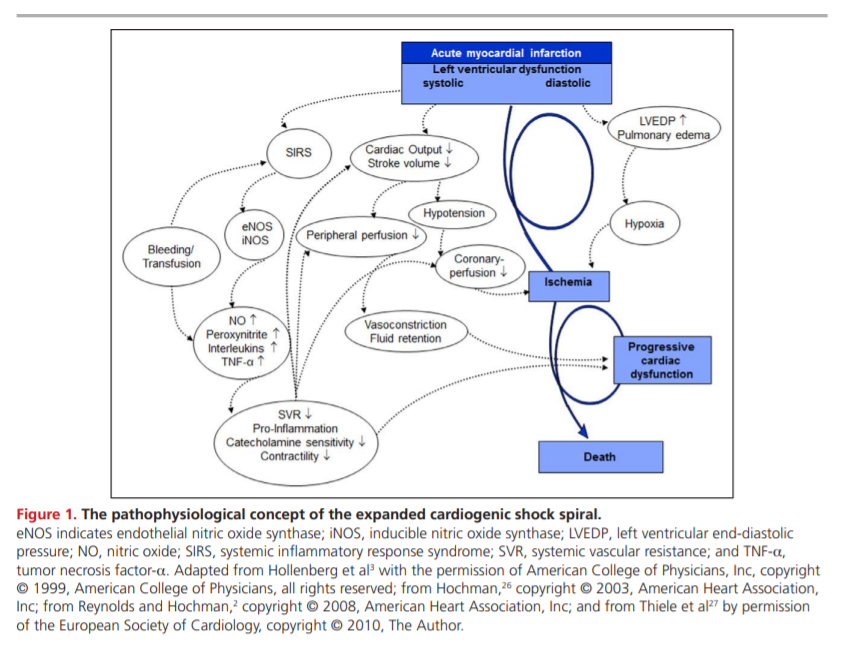
Early reports of CS described patients with HF and elevated central venous pressures (CVPs).34 With the advent of invasive hemodynamic measurements, patients with CS were further characterized by a low CI, an elevated systemic vascular resistance, and a high PCWP.35 This classic “cold and wet” (Figure 2) profile is the most frequent CS phenotype, accounting for nearly two thirds of patients with MI-associated CS.36
Euvolemic or “cold and dry” CS typically describes a diuretic-responsive patient with chronic HF with a subacute decompensation but also represents a reported 28% of patientswith MIassociated CS.36,40 Compared with patients with classic CS, those with euvolemic CS were less likely to have
had a previous MI or chronic kidney disease and had significantly lower PCWPs.36Overlaid on this framework are 2 uncommon but hemodynamically distinct entities of normotensive CS
and right ventricular (RV) CS.In the SHOCK trial registry, 5.2% of patients were normotensive with peripheral hypoperfusion despite an SBP >90 mmHg.50 This group had comparable CIs, PWCPs, and LV ejection fractions but higher systemic vascular resistance compared with hypotensive patients with CS, thus highlighting the risk of relative hypotension and the potential for hypoperfusion without profound hypotension.
The reported prevalence of RV CS is 5.3% among
patients with MI-induced CS. For these patients, the
severity of shock may depend on the degree of both
RV and LV ischemia, given a shared septum and the
importance of ventricular interdependence on RV
function.51–53 Hemodynamically, this cohort is characterized by relatively higher CVPs, LV ejection fractions,
and lower pulmonary artery systolic pressures, with no
differences in CI or PCWP.PATHOGENESIS
After hemodynamic resuscitation and stabilization of a patient presenting with CS, identification of the underlying cause (Supplemental Table 1) can permit the initiation of specific pharmacological or mechanical therapies. A contemporary registry has reported that as many as 81% of patients presenting with CS had an underlying acute coronary syndrome (ACS).55 Thus, among patients with CS within the appropriate
demographic or with risk factors for coronary artery disease, ACS should be the focus of initial diagnostic
testing, and this testing should include an ECG within 10 minutes of presentation.56 Although 5% to 12% of ACS cases are complicated by CS, this presentation is often associated with a large degree of at-risk myocardium.4,57 In patients with a recent ACS, mechanical complications (including papillary muscle rupture, ventricular septal defect, or free wall rupture) were historically thought to be late complications but most frequently present within 24 hours of hospitalization.58,59
An index of suspicion and rapid echocardiography are required for such diagnoses.Chronic HF can present in an acute decompensated state and may account for up to 30% of CS cases.60 These patients have often experienced a decline in disease stability or have poor adherence to guidelinebased therapies that may trigger an acute worsening of their chronic disease. Treatment of patients with
chronic HF presenting in CS can differ substantially from the treatment of other types of CS because the hemodynamic condition and neurohormonal milieu are often strikingly different. Patients with HF often have profound upregulation of vasoconstrictor substances such as angiotensin II, endothelin-1, and norepinephrine.61,62 Among patients who had cardiac surgery, 2% to 6% of patients develop postcardiotomy
shock.63,64 This state may be attributable to low cardiac output (a result in part of myocardial hibernation, stunning, or inadequate cardioprotection), systemic vasodilation, or both.63–65If these common causes of CS are not consistent with the presentation, then less common causes listed in Supplemental Table 1 should be considered. In acute myocarditis, paradoxically, the sickest patients on presentation have the best odds of recovery, particularly in younger age groups.66,67 Survival may depend on rapid recognition of the clinical syndrome and early institution of aggressive hemodynamic support.67–70 Stress-induced cardiomyopathy is increasingly recognized, and although it often presents with mild cardiovascular compromise, it has been associated with CS and may require MCS. Patients with stress-induced cardiomyopathy typically recover.71–73 Advanced valvular heart disease and prosthetic dysfunction, especially when previously undetected or inadequately monitored, may present as CS, although this has become less common as echocardiographic techniques and surveillance have improved.74–76 Thyroid disorders, both hyperthyroidism and hypothyroidism, can also cause circulatory collapse.77,78 Pregnancy-associated cardiac conditions, including both peripartum cardiomyopathy and acute coronary dissection, may present as CS. Numerous additional causes of CS have been reported, but they typically occur in <1% of patients.79,80
LABORATORY EVALUATION, NONINVASIVE TESTING, AND
HEMODYNAMIC MONITORINGSuggestions for Clinical Practice
We suggest that all patients with CS be evaluated with an ECG, chest x-ray, and comprehensive echocardiogram with the specific purpose of understanding the dominant mechanism responsible for acute hemodynamic instability. In the absence of contraindications, additional imaging with a computed tomography scan or transesophageal echocardiogram (as appropriate) if an acute aortic syndrome or pulmonary embolism is suspected is appropriate. Suggested laboratory tests include a complete blood count, electrolytes, creatinine, hepatic function tests, arterial blood gas and lactate, and serial cardiac troponin levels.
CONTEMPORARY OUTCOMES, PROGNOSIS, AND RESOURCE USE
Reperfusion and Revascularization in CS
Coronary reperfusion is the mainstay evidence-based
therapeutic intervention for patients with acute MI
presenting with CS.5,153,154 In this section, reperfusion
and revascularization techniques and other adjunctive
therapies used in the management of CS are reviewed
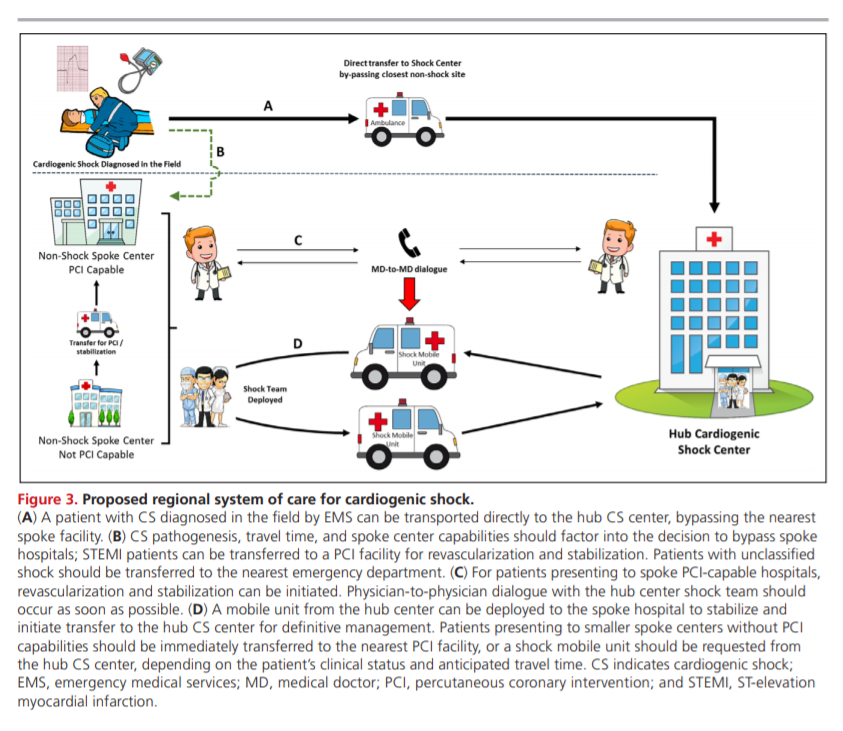
(Supplemental Table 4). A proposed integrated CS care
pathway is outlined in Figure 4.Suggestions/Considerations for Clinical Practice
We suggest that when an early invasive approach cannot be completed in a timely fashion, fibrinolysis can be
considered in CS associated with STEMI. The decision to
administer fibrinolysis should be individualized on the
basis of perceived reperfusion benefit, bleedingEarly Invasive Strategy in CS
Suggestions for Clinical Practice
We support guidelines that recommend an early invasive strategy with appropriate revascularization for all
suitable patients with suspected ACS-associated CS,
including patients with uncertain neurological status or
those who have received prior fibrinolysis, regardless of
the time delay from MI onset.PCI Strategy
Suggestions for Clinical Practice
In summary, evidence continues to support the early
revascularization of patients with CS after ACS, with
either PCI or CABG used as indicated. Until the results
of CULPRIT-SHOCK are available, revascularization of
both the culprit and hemodynamically significant nonculprit stenoses is reasonable. We support the preferential use of radial arterial access for angiography and PCI when feasible.Antithrombotic Pharmacotherapy Adjuncts to PCI
Suggestions for Clinical Practice
We suggest that all patients with CS without serious
bleeding complications be continued on dual antiplatelet therapy without interruption after PCI. In situations
when oral agents cannot be administered or there are
concerns about absorption, the use of an intravenous
glycoprotein IIb/IIIa inhibitor or the recently available intravenous P2Y12 inhibitor cangrelor can be considered.
No high-quality data are available to support the efficacy or safety of glycoprotein IIb/IIIa inhibitors in patients
with MCS.Considerations for Clinical Practice
Overall, the optimal anticoagulation management choice in the setting of PCI for CS remains unclear, and we support following recommendations in the PCI guidelines for patients without CS.176 In patients requiring continued anticoagulation after PCI, we suggest thepreferential use of intravenous unfractionated heparin given the high prevalence of acute kidney injury and acute liver injury in the CS population.
Coronary Artery Bypass
Suggestions for Clinical Practice
We suggest that in patients with MI-associated CS who have multivessel or left main disease, PCI or CABG revascularization decisions should be made collaboratively between cardiologists and surgeons by incorporation of the patient’s medical information, coronary anatomy, procedural risks, potential treatment-related delays, and expressed preferences.
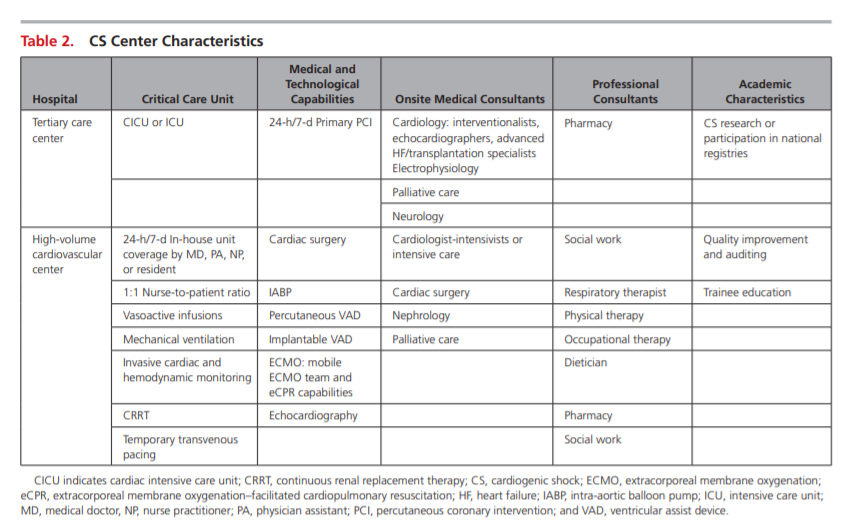
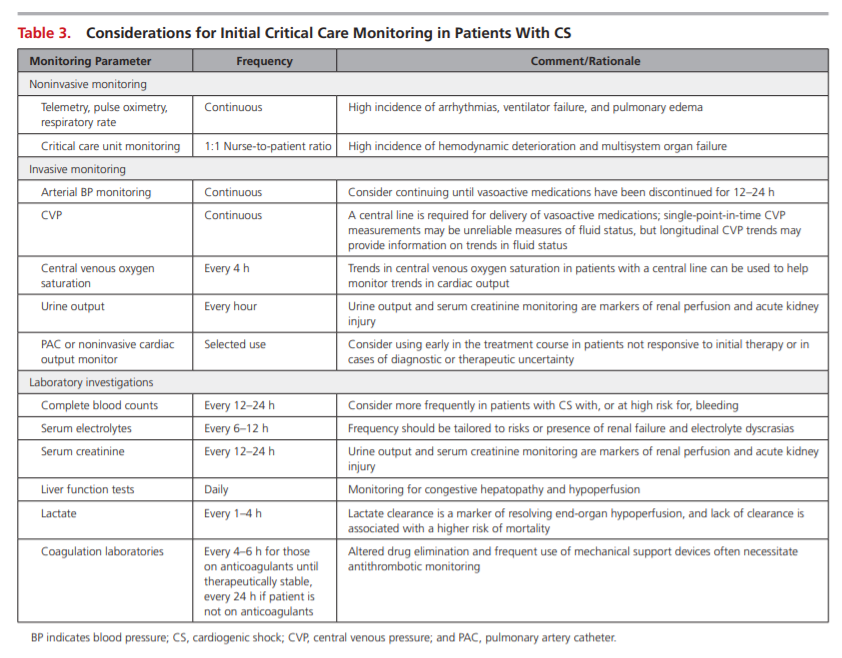
Critical Care Unit Monitoring and Hemodynamic Goals
Relatively few data are available to guide appropriate monitoring decisions for patients with CS. An overview of suggested tools is provided in Table 3.
The inherent hemodynamic instability and high prevalence of vasopressor use in CS merit invasive arterial blood pressure monitoring to guide drug titration. Central venous catheter insertion should also be considered to support the administration of vasoactive medications and to facilitate monitoring of CVP and mixed central venous oxygen saturation,
which may be helpful in determining the adequacy of tissue oxygen delivery. Clinical examination and laboratory testing are also necessary for monitoring end-organ perfusion and function. Repeated assessments of plasma lactate, for instance, can beinformative with respect to the persistence of shock and has been shown to be prognostically important
in patients with CS.198Lastly, although clinical trials have shown no benefit with the routine use of PAC hemodynamic monitoring, observational studies in CS populations have been mixed, and the PAC remains a potentially important diagnostic and management tool for these individuals.199–202 Hemodynamic data provided by a PAC can confirm the presence and severity of CS, involvement of the RV, pulmonary artery pressures and transpulmonary gradient, and vascular resistance of the pulmonary and systemic arterial beds. In addition, a PAC may provide CS prognostic information such as CI and cardiac power and enables clinicians to monitor responses to therapeutic interventions.39,203 Although noninvasive devices may be used, their reliability in this setting has not been well studied.
Although the aforementioned measurements are important for the diagnosis and monitoring of CS, [start here]