In this post I link to and excerpt from the Internet Book of Critical Care [Link is to the Table of Contents] chapter, Atrial Fibrillation In Critical Illness, January 6, 2017 by Dr. Josh Farkas.
All that follows is from the above IBCC chapter:
CONTENTS
All that follows is from the above outstanding chapter, Atrial Fibrillation (AF) & Flutter complicating critical illness. January 6, 2017 by Dr. Josh Farkas:
introduction
what this chapter is about
- AF is the most common arrhythmia encountered in the ICU.(29627355) The two most common scenarios are:
- (1) A patient with chronic AF develops critical illness.
- (2) A patient who was previously in sinus rhythm develops new-onset AF (NOAF) while in the ICU, secondary to the physiologic stress of critical illness (e.g., secondary to sepsis or pulmonary embolism).
- These situations are different from AF in other contexts, for example:
- ICU patients are often hemodynamically tenuous, so they may respond poorly to the usual AF therapies (e.g., diltiazem).
- DC cardioversion alone has a low success rate among critically ill patients (patients will usually revert back into AF).
- The optimal heart rate for critically ill patients is unknown, but some patients may benefit from a mild compensatory tachycardia. Immediately pushing the heart rate down to a “normal” range (e.g., <100) can be dangerous.
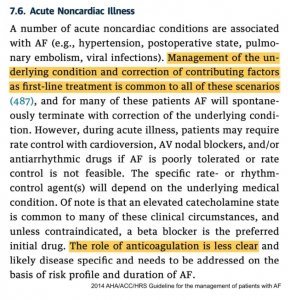
- The below excerpt from the 2014 AHA/ACC guideline on atrial fibrillation provides a nice summary of AF in the context of critical illness.(24685669) Unfortunately, this is all that the AHA/ACC guidelines* have to say about AF in the ICU – so we will have to work to fill in the blanks.
* Here are links to more current guidelines on atrial fibrillation:
- 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC [PubMed Abstract] [Full Text HTML] [Full Text PDF]. European Heart Journal (2020) 00, 1126.
- Summary post on the above2020 ESC Guidelines at Links To And Excerpts From 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS)
Posted on January 19, 2021 by Tom Wade MD - 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons [PubMed Abstract] [Full Text HTHML] [Full Text PDF]. Originally published 28 Jan2019.
diagnosis of AF
general
- AF may be suspected on the basis of an irregularly irregular heart rate (either on clinical examination or telemetry).
- AF diagnosis should always be confirmed with a full 12-lead EKG.
diagnostic criteria for AF on EKG
- [1] There should be no regularity.
- At very high rates, the heart rate may appear to be regular (“pseudo-regularization”).
- When in doubt, calipers may help determine whether there is any regularity.
- [2] No P waves are seen; instead these may be replaced by fibrillation waves.
- Fibrillation waves may be best seen in the inferior and right-sided precordial leads.
- In some patients, fibrillation waves may be small and difficult to distinguish from artifact.
- If it is unclear whether there are P waves or fibrillation waves, consider obtaining a Lewis Lead EKG. Also consider comparison to P wave morphology in prior EKGs (if the patient previously had large, well-defined P-waves and now they’re gone, then this supports an AF diagnosis).
- (One exception to these criteria is that if AF is combined with heart block, then the ventricular response may be regular.)
heart rate among patients in AF
- For most patients who aren’t on medications that suppress the AV node, AF will have a heart rate of ~120-180.
- If the heart rate is >>200, consider the possibility of an accessory tract (AF plus Wolff Parkinson White).
- If the heart rate is <100, conduction disease is likely.
- Be careful when cardioverting patients with a heart rate <100, as there may be an increased risk of bradycardia.
prevention of AF
AF prevention has been studied extensively following cardiac surgery. Most of this literature isn’t applicable to the general ICU population. However, measurement of magnesium levels and repletion may be considered. In the context of post-surgical AF, RCTs have demonstrated that magnesium administration reduced the incidence of AF with an odds ratio of 0.55.(22520937, 23440790) Hypomagnesemia is common among critically ill patients, so detection and treatment of hypomagnesemia makes sense in this population. (More on hypomagnesemia here.)
evaluating the etiology of new-onset AF
common causes of new-onset AF
- Electrolyte abnormalities (especially hypokalemia and hypomagnesemia)
- Toxicology / Medications
- Alcohol (holiday heart syndrome)
- Substance use (especially cocaine, amphetamine, methamphetamine)
- Beta-agonists (norepinephrine, epinephrine, dobutamine, etc.)
- Theophylline
- Fluid overload (yes, fluid is a medication – and sometimes it’s used as a poison)
- Swan-Ganz catheterization
- Adrenergic states
- Alcohol withdrawal
- Pain, agitation
- Primary neurologic disorders (intracranial hemorrhage, ischemic stroke)
- Respiratory failure
- Pulmonary embolism
- Pneumonia, COPD, hypoxemia, hypercapnia
- Myocardial ischemia
- Sepsis
- Thyrotoxicosis
evaluation
- Basic evaluation:
- EKG
- Electrolytes, including magnesium
- Review of medication list
- Review of the presence of any indwelling cardiac devices
- Echocardiogram
- Additional tests as clinically warranted. For example:
- If thoughtful review of EKG and history suggests ischemia, then obtain troponin.
- If there is other evidence suggesting PE, CT angiography may be indicated.
- TSH should be considered if there is no obvious cause of AF, or if other clinical features suggest thyrotoxicosis.
overall approach to AF
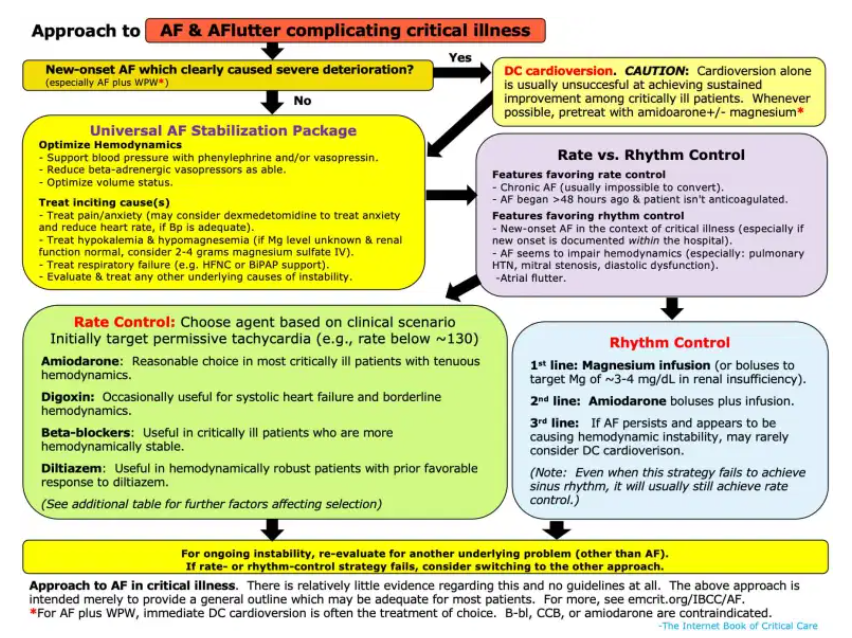
The following figure will serve as a general framework for approaching a critically ill patient with AF:
emergent cardioversion
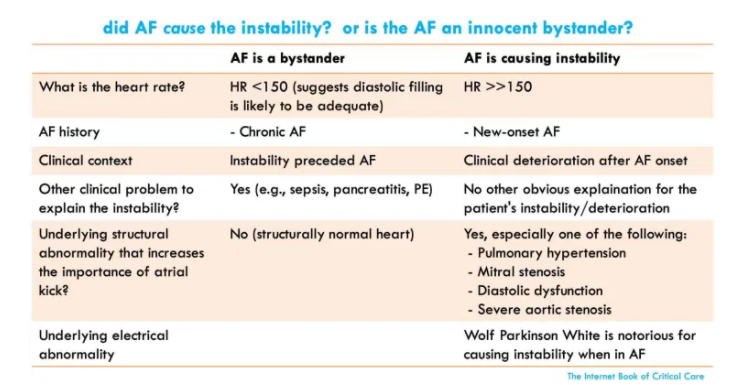
how much is AF actually contributing to the patient’s instability?
- The key question is: What is driving the instability? Is the atrial fibrillation causing the patient to be unstable? Or is atrial fibrillation merely triggered by underlying instability?
- Some key pieces of information can help:
- (1) Heart rate: As a general rule, heart rates <150 are less likely to cause hemodynamic instability. The faster the heart rate is, the more likely it is causing trouble.
- (2) Structural heart abnormalities (especially pulmonary hypertension, mitral stenosis, or diastolic heart failure) may render patients dependent on atrial kick. Such patients may tolerate AF poorly.
- (3) Overall clinical context.
- Trying to sort this out is important:
- DC cardioversion will stabilize the patient only if the AF is causing the instability.
If fast heart rate is due to an underlying process, then overly aggressive attempts to reduce the heart rate to a “normal” range may make matters worse (because a mild degree of tachycardia may actually have a compensatory, beneficial effect).
consider immediate DC cardioversion
- DC cardioversion is indicated if new-onset AF clearly caused the patient to be severely unstable. This is unusual – for most critically ill patients, the AF isn’t the primary driver of instability.
Stand-alone cardioversion will usually fail as a strategy for AF management in critical illness.(12576943) Even if cardioversion is successful, patients will usually revert to AF subsequently.
- If possible, pretreatment or post-treatment with amiodarone +/- magnesium may enhance the likelihood of achieving and maintaining sinus rhythm.
AF with an accessory pathway (Wolff Parkinson White)
- An accessory pathway is an aberrant electrical connection between the atria and the ventricles that shouldn’t exist.
- Normally, when in AF the heart rate is limited by the refractory period of the AV node. Although the AV node may allow for a fast heart rate (e.g. ~120-180), these heart rates are usually tolerated reasonably well.
- When AF occurs in the context of an accessory pathway, both the AV node and the accessory pathway can transmit beats to the ventricles. Since the accessory pathway often has a shorter refractory period than the AV node, it may drive the ventricle very rapidly (e.g. >200). This is dangerous because the extremely fast and uncoordinated contractions of the ventricle can promote ventricular tachycardia or cardiovascular collapse.
- AF with an accessory pathway produces a fairly distinctive pattern of EKG findings:
- Irregularly irregular heart rate that may be extremely fast (e.g. >200).
- Wide-complex beats can result from transmission over the accessory pathway.
- Morphology varies between different beats (some beats are fusion complexes if the AV node and the accessory pathway fire at a similar time).
- AF with an accessory tract shouldn’t be treated with medications that impair the AV node (eg. beta-blockers, calcium channel blockers, or amiodarone). Blockade of the AV node may merely cause a greater dominance of the accessory pathway, exacerbating matters (to a certain extent, the AV node and the accessory pathway are competing for control of the ventricle). Antiarrhythmics which may be used are procainamide or ibutilide.
- This is a unique situation where DC cardioversion is usually the treatment of choice (based on its efficacy and speed). If a patient with AF and an accessory pathway is displaying instability, proceeding directly to DC cardioversion is indicated.
universal AF stabilization package
start here