In this post, I link to and excerpt from “Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations” [PubMed Abstract][Full Text HTML] [Full Text PDF]. Expert Rev Clin Immunol. 2018 Mar;14(3):215-224
Here are excerpts:
Several scoring systems have been designed to quantitate the risk of developing statinassociated muscle symptoms and to manage goal-inhibiting statin intolerance [6,10], but in
clinical practice, it seems reasonable to work with four separate clinical scenarios that have different diagnostic or treatment implications: rhabdomyolysis, myalgia and mild hyperCKemia, self-limited toxic statin myopathy, and the recently described immunemediated necrotizing myopathy. This review describes these conditions, the challenges oftheir diagnosis, and their pathogenesis, with a particular focus on immune-mediatednecrotizing myopathy. Recommendations for clinicians and suggested approaches for
managing these patients are discussed.2. Defining clinical phenotypes
From the clinical viewpoint, patients with statin-associated myopathy can be divided into four groups [11,12]:
- those with rhabdomyolysis,
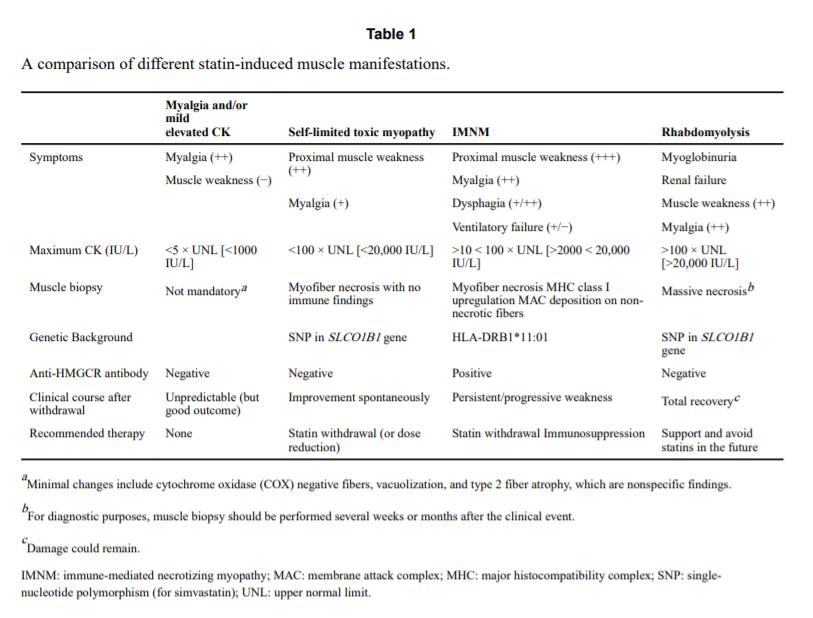
- those with myalgia or mild hyperCKemia
(<5.0 times the upper limit of normal),- those having self-limited toxic statin myopathy, and
- those with myositis (i.e. the recently described immune- mediated necrotizing myopathy with anti-HMGCR antibodies).
4. Clinical recommendations
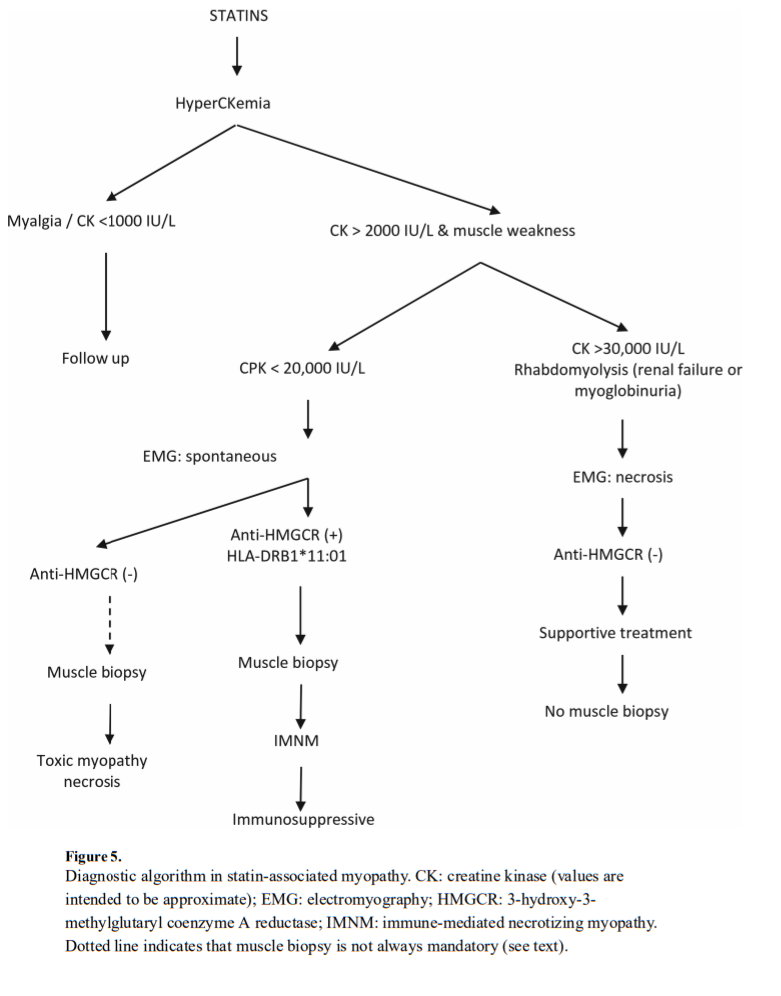
A practical approach to establish clinical recommendations regarding the muscle complaints related to statin use takes into account which of the above mentioned phenotypes best fits a
given patient. Nevertheless, as a set of general rules, it is recommended to first review the indication for statin use in terms of cardiovascular risk, exclude secondary causes of myopathy such as hypothyroidism or the presence of any well-defined myopathy (e.g. McArdle disease or Steinert myotonic dystrophy), and explore whether a high level of physical activity was related with the patient’s signs and symptoms.In a patient with myalgia, serumCK below 1000 IU/L (<5 times the upper limit of normal), and no muscle weakness on physical examination, it is probably safe to maintain the drug and monitor CK levels, which should be stable or have normalized at the next follow-up visit. If CK values rise to more than 10 times the upper limit, the clinician should assess the risk/benefits of drug withdrawal, although it is probably warranted. As a general rule, in a high-risk patient (e.g. previous myocardial infarction) who has myalgia with normal serum CK values and no muscle weakness, we do not recommend withdrawing statin treatment.
When statin administration is associated with rhabdomyolysis, a severe condition, the advice is clear: withdraw the statin and do not administer it in the future. If such as patient is at high
cardiovascular risk, we suggest the use of other, recently approved effective drugs, such as PCSK9 inhibitors [66]. These monoclonal antibodies (e.g. evolocumab, alirocumab, and
bococizumab), which act against the proprotein convertase, have proven effective in reducing cardiovascular events and could be an alternative to statin therapy in severe cases of drug toxicity, such as rhabdomyolysis.It is more difficult to establish clinical recommendations in the other two phenotypes: self limited toxic myopathy and statin-induced autoimmune myopathy. In the first case, drug
withdrawal usually suffices. Muscle biopsy can sometimes be misleading in these patients, and it is important to test for anti-HMGCR autoantibodies to differentiate between the two conditions. If the patient does not improve within 2 weeks after discontinuing the drug and anti-HMGCR antibodies test positive, the diagnosis of immune-mediated necrotizing myopathy is supported and immunosuppressive therapy should be considered. If such a patient is at high cardiovascular risk, clinicians may try to rechallenge the drug, using another statin, a lower dose, or an every-other-day schedule, with strict monitoring of the patient’s CK levels and muscle strength. PCSK9 inhibitors may be an alternative option, as
their mechanism of action is radically different from that of statins, but there is no experience yet in this clinical scenario. Most of the related recommendations are included in Table 1 and the diagnostic algorithm (Figure 5).No guidelines are available for the management of statin-induced muscle disorders. The recommendations reported here are based on our personal experience and are fundamentally
in accordance with guidelines on statin use endorsed by scientific societies or working groups [6,8].Key issues
• Patients with autoimmune myopathy associated with statin use and antiHMGCR antibodies show a pathological picture on muscle biopsy that fits with immune-mediated necrotizing myopathy. Occasionally, a typical inflammatory infiltrate can also be seen. The term statin-induced autoimmune myopathy encompasses this clinical spectrum.
• Statin-naïve patients with anti-HMGCR antibodies and autoimmune myopathy have been described, including children. Other sources of possible statin exposure produced by Aspergillus spp., Penicillium spp., and other molds should be investigated.
• The clinical presentation of statin-induced autoimmune myopathy is heterogeneous. There are mild forms that do not need immunosuppressants and severe forms that are refractory to intense immunosuppression. Clinicians should be aware of this heterogeneity.
• Intravenous immunoglobulin seems to be the best treatment for patients diagnosed with immune-mediated necrotizing myopathy and anti-HMGCR antibodies, and should be included in all therapeutic schemes.
• Immune-mediated conditions such as statin-associated myopathy establish a new paradigm in which drug-related muscle toxicity may modify the HMGCR molecule and trigger an autonomous, autoimmune phenomenon.