In this post, I link to and excerpt from The “5Es” of emergency physician-performed focused cardiac ultrasound: a protocol for rapid identification of effusion, ejection, equality, exit, and entrance [PubMed Abstract] [Full-Text HTML] Full-Text PDF]. Acad Emerg Med. 2015 May;22(5):583-93.
All that follows is from the above resource.
Abstract
Emergency physician (EP)-performed focused cardiac ultrasound (EP FOCUS) has been increasingly recognized as a crucial tool to help clinicians diagnose and treat potentially life-threatening conditions. The existing literature demonstrates a variety of EP FOCUS applications and protocols; however, EP FOCUS is not taught, practiced, or interpreted consistently between institutions. Drawing on over 12 years of experience in a large-volume, high-acuity academic emergency department, we have developed a protocol for teaching and performing EP FOCUS known as “The 5Es,” where each E represents a specific assessment for immediately relevant clinical information. These include pericardial effusion, qualitative left ventricular ejection, ventricular equality, exit (aortic root diameter), and entrance (inferior vena cava diameter and respirophasic variation). Each of these assessments has been well described in the emergency medicine literature and is within the scope of EP-performed echocardiography. This approach provides a reliable and easily recalled framework for assessing, teaching, and communicating EP FOCUS findings that are essential in caring for the patient in the emergency setting.
It has been recognized for more than 25 years that emergency physician (EP)-performed focused cardiac ultrasound (EP FOCUS) is an important skill for the care of patients with potentially life-threatening presentations.1, 2 A recent review detailed 16 specific protocols that included cardiac ultrasound (US) as part of the point-of-care US assessment in medical shock.3 The term “focused cardiac ultrasound” has been addressed in some detail (FOCUS,4 FCU,5 and FoCUS6). However, this term is not specialty-specific, may include assessments that are not relevant in the acute/emergency setting, and has not included assessment of the thoracic aortic root (“exit”), which may be particularly applicable to acute and emergent presentations. We have found that the proximal thoracic aorta can be reliably assessed, providing vital information about potential aortic pathology in patients presenting with acute symptoms.7 We thus propose the “5Es” to assess for the presence of a pericardial effusion, left ventricular ejection, ventricular equality, exit (aortic root diameter), and entrance (inferior vena cava [IVC] diameter and respirophasic variation). The 5Es protocol provides an easy-to-teach, evidence-based, and standardized approach to EP FOCUS for the rapid identification and management of time-sensitive pathologic conditions.
Approach to exam
For images in this article, we will use an emergency medicine convention for cardiac imaging with the probe marker oriented to the patient’s right, which keeps the anatomic right on the screen-left, as is the convention for other US imaging.9 This is in contrast with image and probe orientation utilized in traditional cardiology-performed US, but has been recognized as an accepted convention that we find to be conceptually easier, particularly when performing EP FOCUS as part of an integrated examination such as the focused assessment with sonography for trauma (FAST) or the rapid US for shock and hypotension.3, 6
As has been discussed previously in the emergency US literature, EP FOCUS is not intended to replace comprehensive echocardiography (echo) when more thorough cardiology evaluation is indicated. The clinical questions addressed by EP FOCUS tend to be limited and qualitative but it should be understood that EP FOCUS findings may fall on a spectrum that can make binary categorization challenging. The EP is encouraged to use professional judgment for the interpretation and integration of his or her findings into the diagnosis and care of the patient, as well as the need for specialist consultation.
Effusion
The first “E” in our protocol is an assessment for pericardial effusion. Of the echo components in our protocol, detection of pericardial effusion was the first to be clearly investigated and delineated in the literature and has been incorporated as part of the FAST for more than 20 years.
Techniques for Assessing Effusion
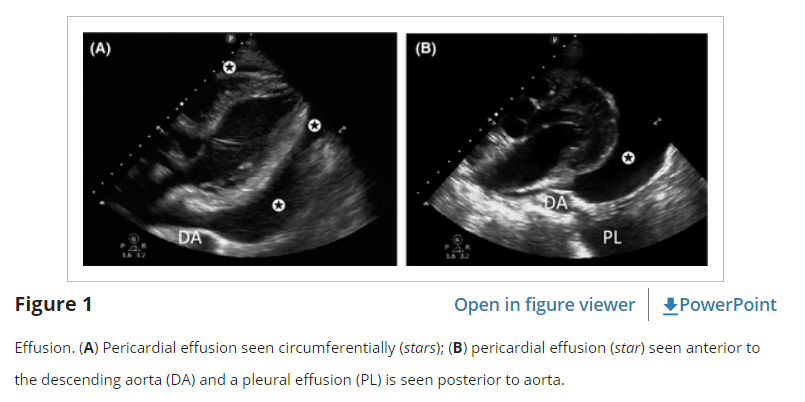
The subcostal window (either SC4C or SCLA) is the most reliable view for detecting pericardial effusions because the most dependent portion of the pericardium is closest to the face of the probe. In this window, the liver can also help provide an acoustic window to the inferior pericardium. In the parasternal views (PSLA and PSSA), significant effusions should be visualized posterior to the left ventricle (LV) and not just anteriorly, as this will often be a fat pad and not an effusion (Figure 1). In the A4C view, small effusions may be visible lateral to the LV free wall, and moderate to large effusions may be seen tracking completely around the apex of the heart.
Normal patients have a trace amount physiologic pericardial fluid that may be seen with modern equipment and described as trivial or “not clinically significant.” True effusions can be categorized as “small,” “moderate,” or “large.” This categorization is often qualitative, although an effusion can be measured by assessing the largest pocket of fluid at end-diastole and measured orthogonally to the surface of the heart. By convention, small effusions are smaller than 1 cm, moderate effusions are 1 to 2 cm, and large effusions are >2 cm.18 Moderate to large effusions are more likely to have an effect on hemodynamics; however, even small effusions can also result in tamponade physiology.19, 20 Prognosis in the setting of pericardial effusion is largely related to time course and etiology, and it is important to recognize that the hemodynamic sequelae of an effusion is much more important than its actual size.18, 21
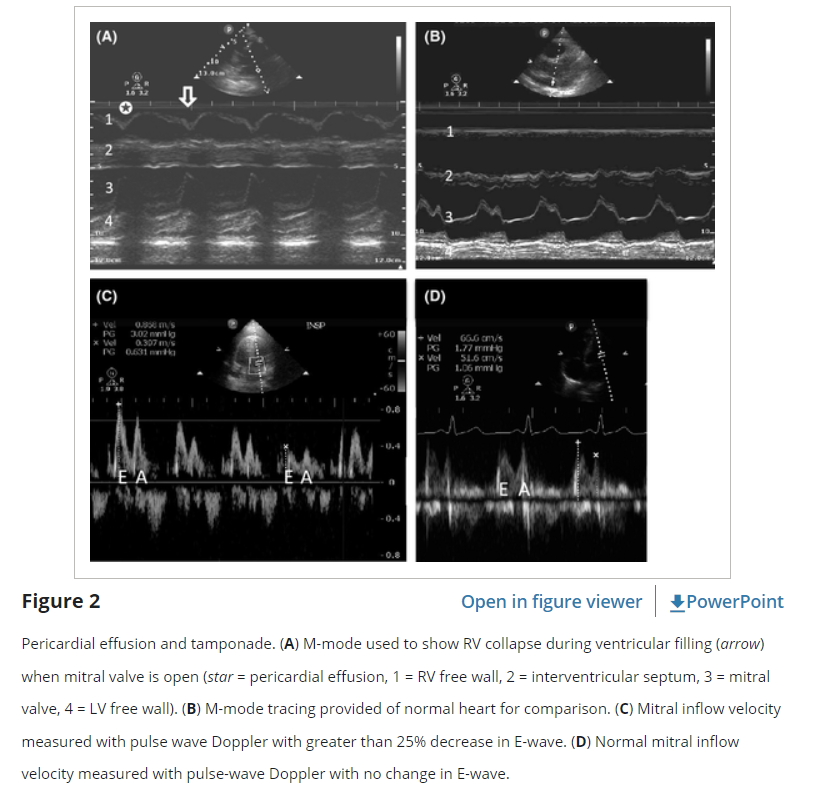
Identification of an effusion should prompt the practitioner to look for signs of tamponade physiology. As pressures inside the pericardium elevate, US will show a progression of findings beginning with collapse of the right atrium (RA), collapse of the right ventricle (RV), and finally LV collapse.20 One of the most easily obtained and sensitive signs of tamponade is the presence of a noncollapsible, plethoric IVC (the fifth “E”), indicating impaired filling from extrinsic compression of the heart.22 Collapse of the RA in ventricular systole or the RV in diastole indicates tamponade physiology, but tachycardia may make it difficult to differentiate normal systolic ventricular collapse from pathologic diastolic ventricular collapse. To confirm RV diastolic collapse, M-mode can be used in either the PSLA or SX4C views to demonstrate RV free wall motion as it relates to the anterior leaflet of the mitral valve (Figure 2). If the RV free wall is collapsed when the mitral (anterior) valve is open, it indicates diastolic collapse of the RV.
Tamponade physiology can also be demonstrated on echo by exaggerated respiratory variation of ventricular in-flow velocities (the echo equivalent of pulsus paradoxus).23 In an A4C view, a pulsed wave spectral Doppler gate is placed at the tips of the mitral valve during diastole. A change of more than 25% in the early filling signal (“E wave”) indicates impaired filling (Figure 2). While these techniques can help determine the presence of tamponade physiology, they are not completely sensitive or specific and should be used in conjunction with clinical judgment.
Pearls and Pitfalls for Effusion
A common pitfall, particularly among novice practitioners of EP FOCUS, is confusing epicardial or pericardial fatty tissue for an effusion.24 Fatty tissue can be characterized by its heterogeneous echo-texture, its coordinated movement in conjunction with the myocardium, and its failure to track around the heart, especially at the apex and posteriorly. A false-positive diagnosis may occur when a hypoechoic space is seen only anterior to the heart on the PSLA view. In the parasternal views, pathologic effusions are typically visible posteriorly, in the most dependent portion of the pericardium (Figure 1). Most clinically significant effusions will not obliterate during diastole and can be traced with US around the apex of the heart and/or posteriorly. Exceptions are loculated or focal effusions and therefore multiple views are recommended.
It should be noted that there are other causes of RA and RV diastolic collapse, including severe hypovolemia and large pleural effusions. Pleural effusions may be misinterpreted as pericardial effusions, particularly in the PSLA window where left-sided pleural fluid lies adjacent to the LV. They can be differentiated by their relationship to the descending aorta. Pericardial effusions will track between the descending aorta and the LV free wall, while pleural effusions will track posterior and lateral to the descending aorta (Figure 1).
Due to the nonspecific clinical presentations of pericardial effusion and tamponade, we recommend having a low threshold for employing US, particularly when a patient presents with unexplained dyspnea, tachycardia, hypotension, near-syncope, or cardiomegaly on chest radiograph. While visualization of the pericardium is essential in the initial evaluation of penetrating thoracoabdominal trauma, pericardial effusion as a result of blunt trauma is rare, and such patients are unlikely to survive to ED presentation.23 The identification of a pericardial effusion in blunt trauma should raise the suspicion of either a false-positive or an incidental pericardial effusion and should not necessarily indicate the need for acute intervention unless there is severe hemodynamic compromise without another source.