In this post I link to and excerpt from Dr. Josh Farkas’ Internet Book Of Critical Care chapter – GI Bleeding, September 18, 2018.
Note to my readers: I just excerpt from my learning resources to help fix the material in my mind. Other readers should just go to Dr. Farkas’ chapter above – as always, it seems to me, Dr. Farkas chapter on any subject is the best resource there is. Here is the link to the Table Of Contents of The Internet Book Of Critical Care.
Dr. Farkas gives us direct links to each section of this outstanding chapter:
CONTENTS
Here are excerpts:
diagnosis & risk stratification
diagnosis of GI bleed:
- Usually easy to diagnose, but consider:
- Posterior epistaxis can cause patients to swallow blood, mimicking an upper GI bleed. This can cause hemorrhagic shock.
- Bloody diarrhea (e.g. due to mesenteric ischemia or infectious colitis) can be misleading. Although this is technically a GI bleed, bleeding isn’t the main problem.
physical exam with bedside ultrasonography
- Hemodynamic evaluation
- Collapsed inferior vena cava and hyperkinetic left ventricle suggest volume depletion from bleeding.
- If a normal or distended IVC is seen in a patient with shock, this argues against hemorrhagic shock as the cause of the patient’s instability. In this scenario, initiating a massive transfusion protocol is probably the wrong move.
- Ascites
- Ascites can easily be evaluated with a FAST exam (or an abbreviated exam including the right & left upper-quadrant FAST views).
- Presence of ascites suggests cirrhosis, with a potential benefit from octreotide and antibiotics. However, note that hemoperitoneum should be considered if there aren’t clear signs of gastrointestinal hemorrhage. Occasionally patients will present with splenic laceration due to trivial trauma or splenic pathology.1 2
- Gastric distension
- Gastric ultrasonography is a simple, validated approach to evaluate gastric size and contents.3
- A collapsed stomach argues against active bleeding in the esophagus or stomach (e.g. variceal hemorrhage). Alternatively, a distended stomach may suggest upper GI hemorrhage if the patient hasn’t recently eaten.4
- For patients undergoing intubation, gastric distension increases the risk of aspiration (more on this below).
risk stratification
Hemoglobin
- Best case scenario: Profound anemia (e.g. hemoglobin <5 g/dL or <50 g/L) in a patient who is hemodynamically stable and minimally symptomatic implies a chronic bleed, with little risk of rapid deterioration. These patients have been bleeding for days, meanwhile gradually retaining volume to compensate (isovolemic anemia). The only immediate danger to these patients is iatrogenic: if given blood too rapidly they will develop volume overload. Ideal management isn’t to slam in several units of blood, but rather to provide gradual transfusion (often in combination with diuresis).
- Worst case scenario: Normal hemoglobin with hemodynamic instability is worrisome for severe bleed. Hemoglobin takes time to fall in response to bleeding, so normal hemoglobin plus shock implies a very active bleed.
- Intermediate scenarios: Many patients will present with moderate anemia (e.g. hemoglobin 6-7 g/dL or 60-70 g/L) and hemodynamic stability. In this case, it can be helpful to determine the patient’s response to blood transfusion. A unit of packed cells should increase hemoglobin by ~1 g/dL (~10 g/L). Failure to respond appropriately to transfusion implies ongoing bleeding.
Hemodynamics
- Hypotension or elevated shock index (HR/SBP above ~0.8) are extremely concerning.
- Syncope or presyncope are worrisome. However, orthostatic vital signs are unhelpful (myth debunked in video below).
- Pressor-dependent shock is the most scary feature. In patients with reasonable hemodynamic reserve, this is an extremely late manifestation that implies profound blood loss. Consider initiation of massive transfusion protocol.
Coagulation
- Presence of easily reversible coagulopathy is a good sign (e.g. markedly supratherapeutic INR from warfarin). If the patient has survived living with an INR of 10, then they will do much better once their INR is normalized. So although the INR elevation can invoke panic, it’s actually a favorable prognostic sign.
- Poorly reversible coagulopathy is worrisome (e.g. NOACs).
Signs of bleeding
- Active bleeding is obviously worrisome. Hematemesis is more worrisome than hematochezia, because upper GI bleeding carries a higher mortality than lower GI bleeding.
- The most concerning is active bleeding from both ends (hematemesis plus hematochezia), as this implies brisk upper GI bleeding with rapid transit through the GI tract.
- Cessation of bleeding (e.g. no recent bowel movements or hematemesis) is reassuring. For example, a patient who hasn’t had a bowel movement in >12-24 hours probably doesn’t have a critical GI bleed (noting that blood is a cathartic).
High-risk conditions
- Cirrhosis is worrisome regarding risk of variceal hemorrhage.
- Prior abdominal aortic aneurysm repair may raise concern for aorto-enteric fistula.
- Overall fitness: Elderly patients with numerous comorbidities are at higher risk of poor outcome.
resuscitation basics
labs
- Chemistries
- CBC (cycle perhaps ~q8hrs)
- PT, PTT, Fibrinogen
- Type & cross-match
- Cirrhotic patients: thromboelastography (TEG) is useful for patients with elevated INR
access
- Two large-bore peripheral IV lines.
- Patients undergoing massive transfusion benefit from central access. Any of the following will work, with my preferences as follows:5
- (1) MAC catheter (multi-lumen access catheter) if you can find one.
- (2) Hemodialysis catheter (large-caliber access, two lumens, doesn’t kink).
- (3) Standard central line (universally criticized due to low flow rates, but if attached to a Level-1 or Belmont rapid infuser it will work just fine here).
- For patients undergoing massive transfusion, consider emergent placement of a femoral arterial line plus femoral venous access (the dirty double). An arterial catheter is invaluable when running a massive transfusion to titrate vasopressors and avoid overshoot hypertension (which may encourage re-bleeding).
blood products
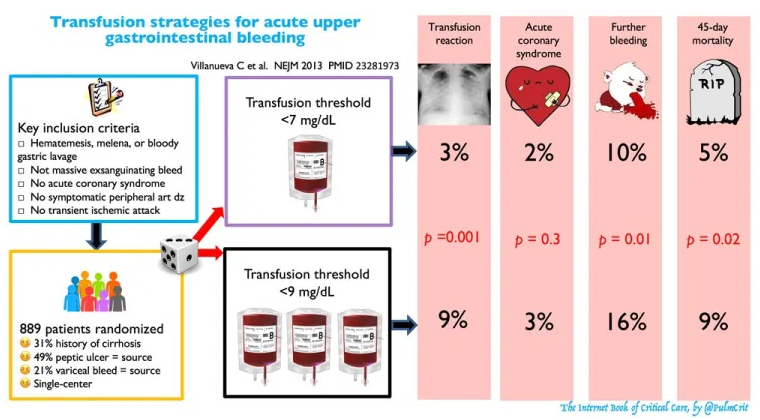
- The hemoglobin target should be > 7 g/dL (>70 g/L) in nearly all cases (infographic below).6 Exceptions include:
- Massive bleed with hemodynamic instability.
- Acute coronary syndrome (target hemoglobin > 8 g/dL or >80 g/L).
- If you’re really worried that the patient will open up, request 4 units PRBC on hold in the blood bank. Don’t transfuse to a high hemoglobin to “tank up” the patient.
- Massive transfusion protocol (MTP): For severe instability (e.g. vasopressor dependence) consider activation of a massive transfusion protocol (link). In this situation, blood products will be provided with a 1:1:1 ratio of PRBC:FFP:platelets. Also consider cryoprecipitate, IV calcium, and a warming blanket.
medications
- Proton Pump Inhibitor: If upper GI hemorrhage possible, give IV proton pump inhibitor. There’s no evidence that a continuous infusion is superior to intermittent IV bolus therapy (e.g. 40 mg pantoprazole IV q12hr).7
- Octreotide: If variceal hemorrhage is possible, give octreotide (50 microgram bolus followed by 50 mcg/hr infusion). This is safe, when in doubt just give it.
- There is even some weak evidence that it might be helpful in non-variceal bleeding.
- Antibiotic: Cirrhosis plus GI bleeding equals antibiotics (usually ceftriaxone 1 gram daily). More on this below.
NG tube with gastric lavage?
- Diagnostic performance:
- Sensitivity for upper GI bleed is ~50%, so negative lavage doesn’t exclude upper GI bleed.
- Bloody lavage has high specificity for an upper GI bleed. Bloody lavage also increases the risk of deterioration and argues for prompt upper endoscopy.
- Historically, gastric lavage has been applied broadly to patients with minimal benefit. For example, if a patient has hematemesis then they obviously have an active upper GI bleed – performing a NG lavage provides no diagnostic information.
- Gastric lavage can be useful in the patient with a probable lower GI bleed, because this helps direct whether the patient should receive an upper endoscopy versus CT angiogram of the abdomen.
- This should be performed within a specific algorithm for approaching hematochezia (more on this below).
- NG tube placement can be useful to therapeutically drain the stomach prior to intubation, to reduce the risk of aspiration.
approach to upper GI bleed