In this post I link to the Internet Book Of Critical Care’s chapter, Unfractionated heparin (UFH), LMWH, fondaparinux, argatroban, and bivalirudin, by Dr. Josh Farkas, May 24, 2020.
Dr. Farkas has given us direct links to each section of his chapter:
CONTENTS
- Overview of indirect-acting anticoagulants (heparin & fondaparinux)
- Unfractionated heparin (UFH)
- Low molecular-weight heparin (LMWH)
- Fondaparinux
- Intravenous direct thrombin inhibitors (DTIs)
- Podcast
- Questions & discussion
- Pitfalls
- PDF of this chapter (or create customized PDF)
Here are excerpts:
basic science
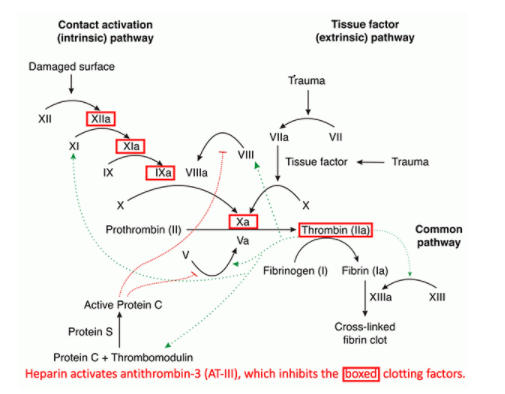
- Unfractionated heparin (UFH) binds to anti-thrombin III (AT-III), which enhances antithrombin’s inhibition of several coagulation factors – especially factor Xa and factor IIa (thrombin).
- Low Molecular-Weight Heparin (LMWH) is a heterogeneous collection of heparin molecules with a lower average molecular weight compared to unfractionated heparin. Since longer length is necessary to facilitate the interaction between anti-thrombin III and factor IIa, LMWH is less effective at inhibiting factor IIa (acting mostly via inhibition of Xa).
- LMWH preparations have differences in the distribution of heparin chain lengths. The ratio of anti-Xa activity to anti-IIa activity varies between preparations (between roughly ~2:1 to ~4:1).
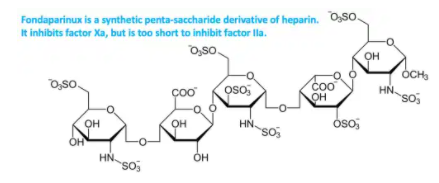
- Fondaparinux is essentially a synthetic, short molecule shaped like heparin. It exerts no activity against factor IIa, working purely via inhibition of Xa.
- All of these agents are effective only against fluid-phase clotting factors. In contrast, direct thrombin inhibitors may inhibit both fluid-phase and clot-bound thrombin. This might theoretically make direct thrombin inhibitors more potent agents.
choice of agent
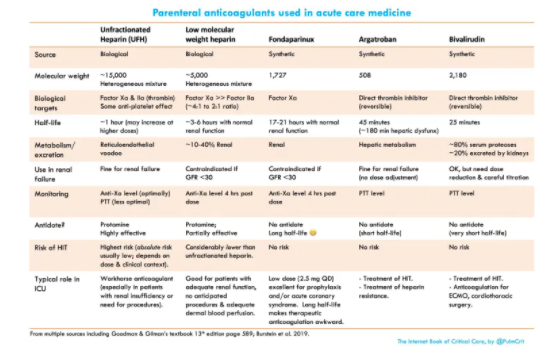
UFH vs. LMWH
- LMWH is generally preferable for the following reasons:
- LMWH is easier to give logistically (doesn’t require IV infusion or monitoring).
- LMWH has a decreased risk of heparin induced thrombocytopenia with thrombosis (HIT).
- Studies comparing UFH and LMWH generally show that LMWH is more effective and causes less bleeding.
- However, UFH is still needed in the following situations:
- Renal failure (GFR < 30 ml/min).
- Need to rapidly stop anticoagulation (e.g., in a patient at risk for bleeding, or pending a procedure).
LMWH vs. Fondaparinux
- LMWH is generally preferable in the ICU because it has a shorter duration of action (half-life of ~4 hours versus ~20 hours). Therapeutic fondaparinux is problematic if the patient starts bleeding or requires an unexpected procedure. Furthermore, fondaparinux has no reversal agent (unlike LMWH, which can be ~50% reversed with protamine).
- Fondaparinux has the advantage of never causing HIT, so:
- Fondaparinux can be used in a patient with a history of HIT.
- Fondaparinux use simplifies the workup for new-onset thrombocytopenia (you don’t need to worry about HIT).
- In general ICU practice, fondaparinux is used mostly at a low (“prophylactic”) dose of 2.5 mg sq. daily.
- May be used for DVT prophylaxis.
- This same dose is preferred therapy for patients with NSTEMI based on the OASIS-5 trial.
risk assessment*
*See this section of the IBCC chapter for details.
dosing & monitoring of unfractionated heparin*
*See this section of the IBCC chapter for details.
heparin resistance*
*See this section of the IBCC chapter for details.
various LMWH agents and dosing*
several LMWH formulations are available (including enoxaparin, dalteparin, and nadroparin)
- The greatest experience exists with enoxaparin, especially in terms of monitoring anti-Xa levels.
- Thus, enoxaparin is a preferred agent – especially in patients with unusual weight or pharmacokinetics.
- However, enoxaparin doses may be roughly correlated into dalteparin doses as follows below.
- Dosing with tinzaparin and nadroparin is less clear, as different formulations may have variable amounts of anti-Xa activity.
understanding dosing differences between enoxaparin and dalteparin
- 1 mg enoxaparin has 100 units of anti-factor Xa activity (e.g., 40 mg enoxaparin = 4,000 units of anti-Xa).
- 1 mg of dalteparin has 156 units of anti-factor Xa activity. Dalteparin is typically measured in terms of anti-Xa units, rather than in milligrams.
- So: (dalteparin dose measured in units)/100 is roughly the equivalent enoxaparin dose in mg. For example, 5000 units of dalteparin is roughly equivalent to 50 mg of enoxaparin.
- However, the ratio of anti-Xa vs. anti-IIa may vary between different medications, so it cannot be assumed that the two drugs are interchangeable.
enoxaparin dosing for full anticoagulation*
*See this section of the IBCC chapter for details.
enoxaparin dosing for DVT prophylaxis*
*See this section of the IBCC chapter for details.
fondaparinux*
*See this section of the IBCC chapter for details.
direct thrombin inhibitors (DTIs)
basic properties
- These act via direct inhibition of thrombin (factor IIa). This has numerous physiologic effects as shown above (e.g., inhibits activation of factors V, VIII, and XI).
- They are active against both fluid-phase and clot-bound thrombin (unlike heparin, which acts only on fluid-phase thrombin).
- These prolong the INR, PTT, and the thrombin time (TT) (because they impair the final common pathway of clot formation).
- PTT prolongation is generally used to titrate the dose of a direct thrombin inhibitor.
- INR prolongation is problematic, as this can make it difficult to transition to warfarin.
- Assays for clotting factors and fibrinogen may be falsely prolonged (causing the lab to register falsely low values).
advantages of direct thrombin inhibitors compared to heparin
- They avoid the issue of heparin-induced thrombocytopenia (or any concerns about this possibility).
- They are not dependent on anti-thrombin III levels
- Pharmacokinetics are generally more predictable than those of heparin (especially bivalirudin, which doesn’t bind to plasma proteins).
- Bivalirudin’s short half-life (25 minutes) may make it easier to stop, compared to a heparin infusion (with half-life close to 45 minutes).
disadvantages compared to heparin
- Less widespread experience and less universally available.
- Lack of any reversal agent. This could be problematic for patients with hepatic dysfunction on argatroban – wherein the half-life may be considerable.
selection of different agents
- Argatroban has traditionally been used more in the ICU (especially as a front-line agent for patients with HIT or suspected HIT).
- Bivalirudin has traditionally been used more in the cardiac catheterization laboratory. More recently, bivalirudin has been used increasingly in ICUs as an anticoagulant for ECMO.
- Properties of the two agents are compared here:
direct thrombin inhibitor pseudo-resistance*
*See this section of the chapter for details.
argatroban
basics
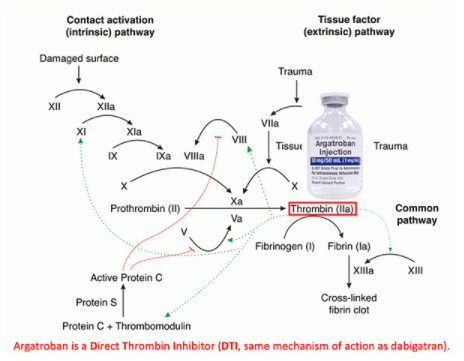
- Argatroban is a small molecule which directly inhibits thrombin (factor IIa)(figure above).
- Argatroban is cleared by the liver with a ~45-minute half-life. In patients with normal hepatic function, coagulation will normalize about 2-4 hours after stopping the infusion. The half-life may extend to ~3 hours in patients with hepatic dysfunction. Argatroban may be used in patients with liver dysfunction, but lower doses should be used and it may be harder to rapidly discontinue anticoagulation
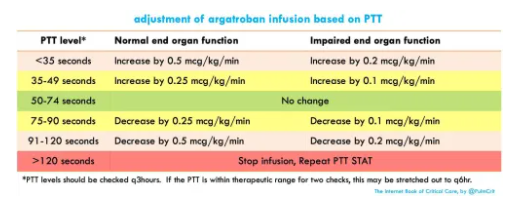
dosing & monitoring
- For ICU patients with multi-organ failure (and especially with hepatic dysfunction) a low starting dose is safest (~0.3 ug/kg/min). In patients with otherwise intact organ function, a higher starting dose may be considered (~1.5 ug/kg/min).
- Use caution in patients with hepatic dysfunction (e.g. serum bilirubin >1.5 mg/dL)(Sedhai et al 2020).
- Titration is based on the PTT value, with a goal PTT of ~1.5-2.5 times normal (table below).
- Make sure to obtain a baseline PTT before starting the argatroban infusion (and possibly a thrombin time as well)(Beiderlinden et al. 2018).
- If the PTT is elevated at baseline and this is unrecognized, it may lead to sub-therapeutic argatroban dosing (“PTT confounding”).
- If the PTT is reduced at baseline, this could lead to problems with argatroban pseudo-resistance (discussed below).
- The optimal target PTT might be 1.5-3 times the baseline value (Sedhai et al 2020). However, sources disagree about whether to target PTT to the patient’s baseline or to the laboratory baseline.
- Medscape monograph on argatroban.
bivalirudin
basics
- Bivalirudin is a synthetic 20-amino acid long peptide designed to mimic the active site of hirudin, an anticoagulant produced by the medicinal leech Hirudo medicinalis. Along with argatroban, it is an intravenous direct thrombin inhibitor.
- Given its peptide structure, bivalirudin is partially metabolized by serum proteases. Additionally, ~20% of its clearance is due to renal excretion (Bohman et al. 2019). Dose adjustment is necessary in renal insufficiency.
- Cleavage by serum proteases may help achieve stable serum levels in the face of fluctuating organ function. However, this can also lead to pockets of low bivalirudin levels in regions of low-flow, stagnant blood, which may lead to thrombus formation (which is mostly an issue with ECMO and cardiac surgery).
dosing & monitoring
- The initial infusion rate may vary depending on renal function (see table below). Maintenance infusion rates typically range from ~0.05 mg/kg/hr to 0.25 mg/kg/hr (Burstein et al. 2019).
- Use of a loading bolus may be important for some applications (e.g., emergency percutaneous coronary intervention or cardiothoracic surgery). However, for use as a maintenance anticoagulant in the ICU there is no clear requirement for a loading bolus.
- Doses >0.5 mg/kg/hr should be avoided; the requirement for such high doses may suggest pseudo-resistance (see pseudo-resistance in the section on direct thrombin inhibitors)(Walker et al 2019).
- Protocols:
- When available, local protocols should be utilized.
- A protocol from Yale is shown below (Cardinale et al. 2016). An identical protocol is used by the University of Washington (here and here).
- Titration is based on the PTT value, with a goal PTT of ~1.5-2.5 times normal (table below)
podcast*
*Click on the above link to go to the podcast in the IBCC chapter.
Pitfalls
- Don’t give enoxaparin or (especially) fondaparinux to a patient who may need a procedure soon (especially lumbar puncture).
- Avoid antithrombin administration among patients with heparin resistance and borderline anti-thrombin-III levels (such patients often have multi-factorial heparin resistance, so anti-thrombin III won’t work well).
- Beware of pseudo-heparin, pseudo-argatroban, and pseudo-bivalirudin resistance!
- Be careful of patients on multiple anticoagulants, or patients with multiple risks for bleeding (you can often get away with a single anti-clotting agent, but risk of bleeding multiples with additional agents).
- When in doubt, don’t hesitate to seek help (especially from critical care pharmacists).
Going further: