Today, I review, link to, and excerpt from Ordering Genetic Testing [for cognitive impairment] from UCSF Weill Institute for Neurosciences [accessed 12-20-2024].
All that follows is from the above resource.
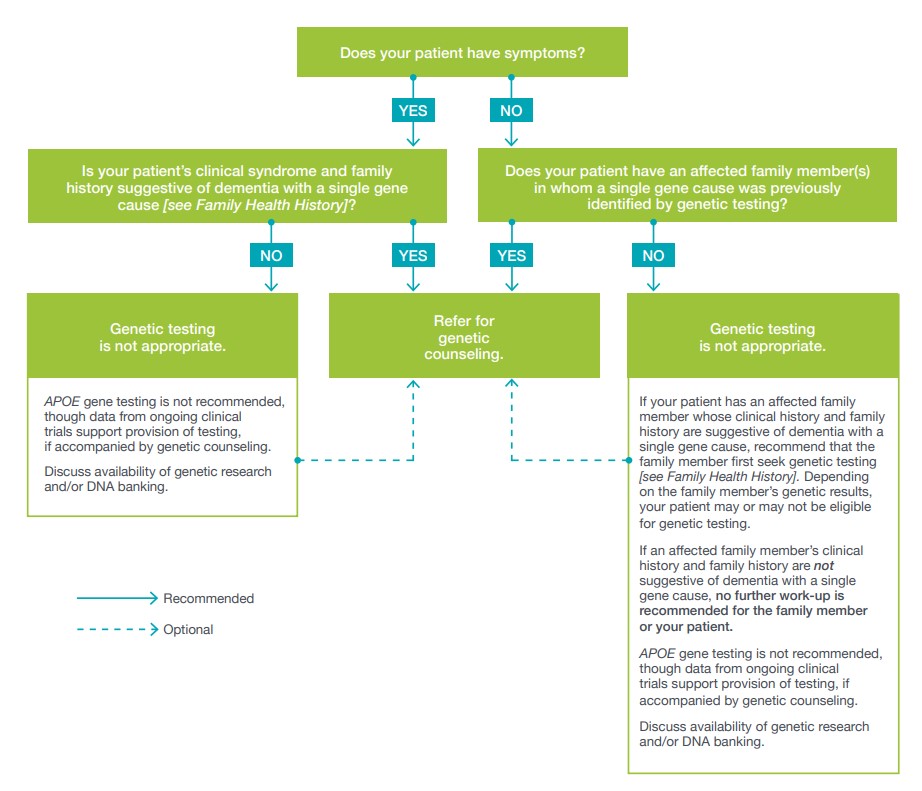
When is dementia genetic testing appropriate for my patient?
You might consider genetic testing for your patient if the following concerns arise:
• You are concerned your patient has a genetic form of dementia
• Your patient has a family member who was diagnosed with a genetic form of dementiaGenetic counseling is recommended before ordering any genetic test so that patients can understand the implications for all family members and can give proper informed consent. A genetic counselor or other genetics professional can provide genetic counseling. Either should have expertise in the genetics
of dementia.When is dementia genetic testing not appropriate for my patient?
Dementia with single gene etiology is rare. This makes genetic
testing appropriate for a small proportion of individuals only.
Genetic testing for pathogenic variants is warranted in certain
situations. Genetic testing for risk variants is not recommended,
even if a test is clinically available. Consider first whether or
not your patient has symptoms.Is APOE gene testing recommended?
The American College of Medical Genetics and Genomics (ACMG) and the National Society of Genetic Counselors (NSGC) recommend against APOE gene testing for your patients with no cognitive symptoms.
The APOE gene encodes a protein called apolipoprotein E (APOE), which is involved in lipid transport and serves as a scaffold for high-density lipoproteins (HDL). It is highly expressed in the liver and the CNS. APOE transports lipids in the body. It is also involved in the transport of amyloid beta, one of the pathological hallmarks of Alzheimer’s disease. The APOE gene has three common versions (alleles), called ɛ2, ɛ3, and ɛ4, which result in six possible genotypes. Frequencies of the three alleles vary, depending on ethnicity. Among Caucasians the most common APOE allele is ɛ3, followed by ɛ4 and then ɛ2. The ɛ4 allele is a risk factor for Alzheimer’s disease, with one or two copies conferring increased lifetime risk to develop the disease. The ɛ2 and ɛ3 variants do not increase risk for Alzheimer’s disease. Depending on ethnicity, about 15–20% of individuals in the general population carry one ɛ4 allele. This frequency increases to about 50% among individuals with Alzheimer’s disease.
Without reference to APOE, the lifetime risk among Caucasians todevelop Alzheimer’s disease is 10–11% for men and 14–17% for
women who live to age 85 years. Having one ɛ4 allele increases
risk by 2-fold, approaching 23% for men and 35% for women who live to age 85 years. Having two ɛ4 alleles increases risk by
4- to 5-fold, approaching 52% for men and 68% for women who
live to age 85 years. These risk estimates do not hold true for all
populations, as allele frequencies and magnitude of risk conferred by the ɛ4 allele differ among ethnic groups. The ɛ4 allele decreases the age of onset of first symptoms. However, the ɛ4 allele does not cause Alzheimer’s disease directly. Overall risk of Alzheimer’s disease for your patient depends instead on a multitude of genetic, epigenetic, and environmental factors.Among your patients with mild cognitive impairment, APOE
ɛ4 carrier status by itself does not predict cognitive decline or
conversion to Alzheimer’s disease. While APOE ɛ4 carrier status is considered by some clinicians a supportive adjunct to the clinical diagnosis of Alzheimer’s disease, current data to support such use are lacking.Research suggests that the recommendation against APOE
genotyping appears to contrast with lay attitudes, which reveal
broad support of learning genetic susceptibility for Alzheimer’s
disease. This is reflected by the large number of customers of
direct-to-consumer (DTC) genetic testing companies offering APOE genotyping. Many consumers of DTC APOE testing are not dissuaded by its poor clinical utility. Many people pursue testing citing a desire to have information for future care and/or financial planning. Many cite a desire to provide information to family members. People may be more likely to obtain APOE genotyping if they can afford to pay for the test.Researchers have begun to examine the effects of disclosing APOE genotype to individuals with no cognitive symptoms in a controlled setting. The Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study, a series of multi-center randomized clinical trials collectively including over 1,100 individuals, demonstrated in a subset of people that APOE genotype and Alzheimer’s risk information can be provided after counseling and education without undue stress and misunderstandings. However, these study groups were small and selective. They excluded people with severe mood
disorders and people with mild cognitive impairment. The study’s findings may not apply to the general population.Take-home message
Many people worry about Alzheimer’s disease. As a concerned
physician, you, together with your patient, will face questions about the patient’s risk to develop Alzheimer’s disease. Although APOE testing is clinically available, it is not advised. Direct-to-consumer APOE genotyping is also not advised [Goldman 2011]. APOE is a susceptibility gene, not a deterministic one. If your patient wishes to pursue APOE genotyping despite recommendations to the contrary, consider referring to a genetic counselor. Genetic counselors who are knowledgeable about dementia can provide counseling and education to your patients. The REVEAL trials suggested that genetic counselors are well suited to help your patients cope and adapt to the short-term emotional distress that may accompany genetic testing [Green 2014].What research about the genetics of Alzheimer’s
disease would be available to my patient?The following resources provide information about
Alzheimer’s disease research:• The Alzheimer’s Association, alz.org
• Clinical Trials, clinicaltrials.govWhat is DNA banking?
DNA banking is the long-term storage of a person’s genetic
material for possible future genetic testing. DNA is most frequently extracted from blood, but can also be obtained from cheek cells, saliva, or other tissues. Numerous commercial laboratories will store DNA for a fee during a pre-determined period of time. Once preserved, DNA can be sent for genetic testing at any time. There may be additional costs associated with transfer of DNA and subsequent genetic testing. DNA can be banked at any time during a person’s life, but may be urgent for the elderly and terminally ill. Once a person is buried or cremated, it may be difficult or impossible to obtain a DNA sample.Reasons your patients and their families might consider DNA
banking are as follows:• Your patient or your patient’s relative has dementia, and death is imminent, leaving no time for standard genetic evaluation or testing, if appropriate.
• Your patient or your patient’s relative has dementia, and
next-of-kin or legally authorized representative and other
family members cannot agree on decisions about genetic
testing, if appropriate.
• Limitations of current technology or disease knowledge have impeded the identification of a genetic cause of what appears to be an inherited dementia in your patient’s family. DNA banking allows your patient’s family to benefit from future scientific advances.The online resource Gene Tests summarizes various commercial
laboratories that offer DNA banking:• Gene Tests, genetests.org/tests
UCSF Weill Institute For Neurosciences
Memory And Aging Center